Copper Chemistry
History
Information on
the history of Copper
is available at the Copper
Development Association, Inc where they make the point that:
" For nearly 5000 years copper was the only metal known to man.
Today it is one of the most used and reused of our modern
metals."
Humans first used copper about 10,000 years ago. A copper
pendant discovered in Northern Iraq is thought to date back to
around 8700 BC. Prehistoric man probably used copper for weapon
making. Ancient Egyptians too seemed to have appreciated the
corrosion resistance of the metal. They used copper bands and
nails in ship building and copper pipes were used to convey
water. Some of these artifacts survive today in good condition.
An estimate of the total Egyptian copper output over 1500 years
is 10,000 tons.
Years later, copper alloys appeared. Bronzes (copper-tin alloys)
came about first followed much later by brass (copper-zinc
alloys). The " Bronze Age" saw the extensive use of copper and
bronze for arms, coins, household utensils, furniture and other
items. The earliest known example of brass use is a Roman coin
minted during the reign of Augustus 27 BC- AD 14. Copper later
played an important role in the advent of electricity and today
is still among our most valued materials.
Usage of copper compounds also dates back to before 4000 BC.
Copper sulphate for example was an especially important compound
in early times. Ancient Egyptians used it as a mordant in their
dyeing process. The compound was also used to make ointments and
other such preparations. Later, medicinal use of copper sulphate
came about with its prescription for pulmonary diseases. Copper
sulphate is still extensively used today and no harmful side
effects of its prescribed use have been reported.
Occurrence
Copper is the earth's 25th most abundant element, but
one of the less common first row transition metals. It occurs as
a soft reddish metal that can be found native as large boulders
weighing several hundred tons or as sulphide ores. The latter are
complex copper, iron and sulphur mixtures in combination with
other metals such as arsenic, zinc and silver. The copper
concentration in such ores is typically between 0.5-2%.
The commonest ore is chalcopyrite, CuFeS2, a brass
yellow ore that accounts for approximately 50% of the world's
copper deposits. Numerous other copper ores of varying colours
and compositions exist. Examples are malachite,
Cu2CO3(OH)2, a bright green ore,
and the red ore cuprite, Cu2O.
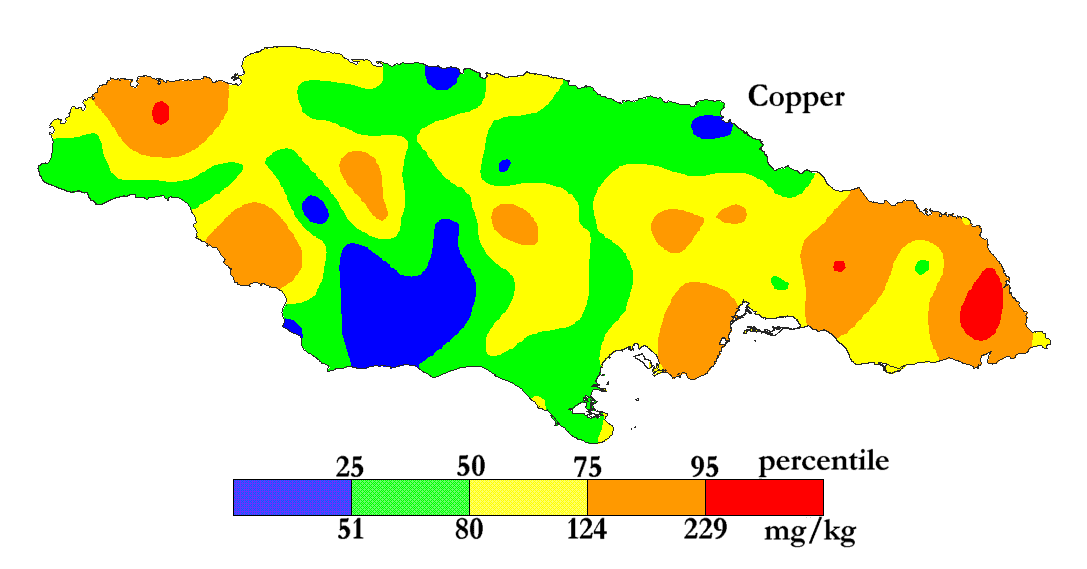
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Copper are shown below (courtesy of Prof G.C. Lalor).
Copper occurs in biological systems as a part of the
prosthetic group of certain proteins. For examples of copper
containing proteins see the article originally from the University
of Leeds, Department of Biochemistry and Molecular Biology at the Scripps Institute.
The red pigment in the
softbilled T(o)uraco Bird
contains a copper porphyrin complex. The pigment is highly water
soluble under alkaline conditions and it was
reported in 1952
that attempts by zookeepers to wash a bird resulted in the water
becoming tinged with red.
T(o)uracos are said to be the only birds to possess true red and green color.
Generally, the color you perceive when observing birds, is due to reflections
produced by the feather structure. The red and green pigments (turacin and turacoverdin)
found in the feathers of the T(o)uraco both contain copper.
Properties of copper
An excellent site for finding the properties of the elements,
including copper is at 
Another useful link is to the
Geology Project pages
at the Univ. of Nevada, Reno.
The Extraction of Copper
Copper is extracted from its ore by two principal methods:
- Pyrometallurgical method
- Hydrometallurgical method
Pyrometallurgical Method
This technique is often used in the extraction of sulphide ores.
There are four main stages:
- Mining and Milling
- The ore is crushed and ground into a powder
usually containing less than 1% copper.
Minerals are concentrated into a slurry that is about 15%
copper.
Copper minerals are separated from useless material by flotation
using froth forming solutions.
- Smelting
- Smelting of the copper concentrate and extraction
by heat, flux and addition of oxygen. Sulfur, iron and other
undesirable elements are removed and the product is called
blister copper.
- Refining
- This is the final stage in the process for
obtaining high grade copper. Fire and electro-refining methods
are the techniques used. The latter produces high purity copper
fit for electrical uses.
Hydrometallurgical Method -SX/EW
Solvent Extraction / Electrowinning is the most dominant leaching
process used today in the recovery of copper from chemical
solutions. As the name suggests the method involves two major
stages:
Solvent Extraction- the process by which copper ions are leached
or otherwise extracted from the raw ore using chemical
agents.
Electrowinning- electrolysis of a metal ion containing solution
such that Cu ions within it are plated onto the cathode and
thereafter removed in elemental form.
The process takes place in the following steps:
- A lixivant (leaching solution) is selected for use in
leaching Cu ions from the ore. Common reagents are weak acids
e.g. H2SO4, H2SO4 +
Fe2(SO4)3, acidic chloride
solutions e.g. FeCl2, ammonium chloride and ammonium
salt compositions.
- When applied to the ore the chosen lixivant dissolves the
copper ions present to give a lixivant product called a "pregnant
leach solution".
- An organic extractant is then selected to remove Cu ions from
the aqueous solution. Preferred organic extractants consist of
hydroxyphenyl oximes having the basic chemical formula:
- C6H3 (R)(OH) CNOHR*, R=
C9H19 or C12H25 and
R*= H, CH3, or C6H5
|
5-nonylsalicylaldoxime structure
|
Examples of such extractants are 5-nonylsalicylaldoxime and a
mixture of this compound and 2-hydroxy-5-nonyl-acetophenone
oxime. The commercially available reagents usually contain 5%-10%
of the oxime in a 90-95% petroleum dilutant such as
kerosene.
Prior to mixing with lixivant product the extractant will
contain little or no copper and is at this stage called the
"barren organic extractant".
- Copper ions are transferred from the leaching solution to the
organic extractant upon mixing of the two reagents. A phase
separation takes place to give an aqueous and an organic phase
termed the first aqueous and first organic phases respectively.
The first aqueous phase, the "raffinate", is the lixivant
stripped of its copper ions while the first organic phase is the
"loaded organic extractant" i.e. extractant with copper ions
present.
- The raffinate is recycled to the leaching pad while the
loaded organic extractant is mixed with an electrolyte solution
called the "lean electrolyte" (i.e. containing no copper).
Typical electrolytes are acidic solutions such as sulphuric acid,
H2SO4. The copper ions that were present in
the organic extractant thus dissolve in the electrolyte solution
to give a copper containing "rich electrolyte." Here again there
is a phase separation. The second organic phase is the barren
organic extractant while the second aqueous phase is the "rich
electrolyte". The barren organic extractant is then recycled for
reuse in application to lixivant product.
- The final stage of the process is the electrolysis of the
acidic metal ion solution. As a result dissolved copper ions
become plated onto the cathode and elemental copper is removed.
The recovery process is thus complete.
A Note on Impurities
The presence of suspended contaminants within a SX/EW system can
significantly compromise its operating efficiency. Such
contaminants may be introduced into the system from the ore or
from the surroundings. The system is susceptible to contamination
from rain, wind and other environmental forces since the first
containment vessel, which stores lixivant product, is typically
uncovered and located outdoors. Thus solid waste material in the
form of dirt, sand, rock dust, vegetable matter, mineral residue
and suspended solids is often introduced into the system in the
early stages and persists in the subsequent stages of the
process.
The effects of these contaminants are considerable and
include:
- increased phase separation time at stages when organic and
aqueous solvents are mixed.
- lack of complete phase separation after extraction,
this results in loss of expensive organic extractant since much
of it remains within the aqueous solution.
- a decrease in the current efficiency and reduction in the
purity of the plated copper product in the electrolysis
stage.
In most SX/EW systems purification steps have been introduced in
order to alleviate this problem. In
US patent (number 573341)
for example, at least a portion of
the second organic phase is filtered to remove solid contaminants
before reuse in treating lixivant product. The recycled organic
extractant therefore contains little or no impurities dependent
on whether a portion or the entire second organic phase was
filtered. It has been found that this filtration step
considerably improves the operating efficiency, even when only a
portion of the extractant is treated.
Uses of copper and its compounds
Copper is second only to iron in its usefulness down the ages.
The metal and its compounds are used in every sphere of life from
the electrical to medicinal and agricultural industries.
Uses of copper metal
The electrical industry is the beneficiary of most of the world's
copper output. The metal is used in the manufacture of electrical
apparatus such as cathodes and wires.
Other uses include:
-Roofing
-Utensils
-Coins
-Metal work
-Plumbing
-Refrigerator and Air Conditioning coils
-Alloys e.g. bronze, brass
Uses of copper compounds
Copper compounds have their most extensive use in Agriculture.
Since the discovery of their toxicity to certain insects, fungi
and algae these compounds have been used in insecticides,
fungicides and to prevent algal development in potable water
reservoirs. They are therefore used in the control of animal and
plant diseases. Fertilisers are also often supplemented with
copper compounds, e.g. copper sulphate, in order to increase soil
fertility and thus boost crop growth.
Copper compounds are also used in photography and as colourants
for glass and porcelain.
Copper for Good Health
Copper is one of many trace elements required for good health. It
is part of the prosthetic groups of many proteins and enzymes and
thus is essential to their proper function. Since the body can
not synthesize copper it must be taken in the diet. Nuts, seeds,
cereals, meat (e.g. liver) and fish are good sources of
copper.
Copper has also found medicinal use. It has been used from early
times in the treatment of chest wounds and water purification. It
has recently been suggested that copper helps to prevent
inflammation associated with arthritis and such diseases.
Research continues into medicines containing copper for treatment
of this and other conditions.
For more information on the importance of copper to health and
copper deficiency see:
MotherNature and/or Vitamin Research
Products
Copper Compounds
Copper exhibits a variety of compounds, many of which are
coloured. The two principal oxidation states of copper are +1 and
+2 although some +3 complexes are known. Copper(I) compounds are
expected to be diamagnetic in nature and are usually colourless,
except where colour results from charge transfer or from the
anion. The +1 ion has tetrahedral or square planar geometry. In
solid compounds, copper(I) is often the more stable state at
moderate temperatures.
The copper(II) ion is usually the more stable state in aqueous
solutions. Compounds of this ion, often called cupric compounds,
are usually coloured. They are affected by
Jahn Teller distortions
and exhibit a wide range of stereochemistries with
four, five, and six coordination compounds predominating. The +2
ion often shows distorted tetrahedral geometry.
Copper Halides
All of the copper(I) halides are known to exist although the
fluoride has not yet been obtained in the pure state. The cuprous
chlorides, bromides and iodides are colouless, diamagnetic
compounds. They crystallize at ordinary temperatures with the
zinc blende structure in which Cu atoms are tetrahedrally bonded
to four halogens. The copper(I) chloride and bromide salts are
produced by boiling an acidic solution of copper(II) ions in an
excess of copper. On dilution, the white CuCl or the pale yellow
CuBr is produced. Addition of soluble iodide to an aqueous
solution of copper(II) ions results in the formation of a
copper(I) iodide precipitate, which rapidly decomposes to Cu(I)
and iodine.
The copper(I) halides are sparingly soluble in water and much of
the copper in aqueous solution is in the Cu(II) state. Even so,
the poor solubility of the copper(I) compounds is increased upon
addition of halide ions. The table below shows some properties of
copper(I) halides.
Copper(I) halides
| Formula |
Colour |
MP |
BP |
Structure |
| CuCl |
white |
430 |
1359 |
- |
| CuBr |
white |
483 |
1345 |
- |
| CuI |
white |
588 |
1293 |
Zinc Blende |
Prepared by reduction of CuX2 -> CuX;
except for the F which has not been obtained pure.
Copper(II) halides
| Formula |
Colour |
MP |
BP |
m (BM) |
Structure |
| CuF2 |
white |
950decomp |
- |
1.5 |
|
| CuCl2 |
brown |
632 |
993decomp |
1.75 |
CdCl2 |
| CuBr2 |
black |
498 |
- |
1.3 |
|
All four copper(II) halides are known although cupric iodide
rapidly decomposes to cuprous iodide and iodine. The yellow
copper(II) chloride and the almost black copper(II) bromide are
the common halides. These compounds adopt a structure with
infinite parallel bands of square CuX4 units. Cupric
chlorides and bromides are readily soluble in water and in donor
solvents such as acetone, alcohol and pyridine.
Copper(II) halides are moderate oxidising agents due to the
Cu(I)/ Cu(II) couple. In water, where the potential is largely
that of the aqua-complexes, there is not a great deal of
difference between them, but in non-aqueous media, the oxidising
(halogenating) power increases in the sequence; CuF2
<< CuCl2 << CuBr2.
They can be prepared by direct reaction with the respective
halogens:
Cu + F2 → CuF2;
Cu + Cl2 / 450 C → CuCl2;
Cu + Br2 → CuBr2
Alternatively they can be prepared from CuX2.aq by
heating -> CuX2
Copper Oxides
Copper(I) oxides are more stable than the copper(II) oxides at
high temperatures. Copper(I) oxide occurs native as the red
cuprite. In the laboratory, the reduction of
Fehling's solution
with a reducing sugar such as glucose produces a red precipitate.
The test is sensitive enough for even 1 mg of sugar to produce
the characteristic red colour of the compound. Cuprous oxide can
also be prepared as a yellow powder by controlled reduction of an
alkaline copper(II) salt with hydrazine. Thermal decomposition of
copper(II) oxide also gives copper(I) oxide since the latter has
greater thermal stability. The same method can be used to prepare
the compound from the copper(II) nitrate, carbonate and
hydroxide.
Copper(II) oxide occurs naturally as tenorite. This black
crystalline solid can be obtained by the pyrolysis of the
nitrate, hydroxide or carbonate salts. It is also formed when
powdered copper is heated in air or oxygen. The table below shows
some characteristics of copper oxides.
Copper oxides
| Formula |
Colour |
Oxidation State |
MP |
| CuO |
black |
Cu2+ |
1026decomp |
| Cu2O |
red |
Cu+ |
1230 |
Redox Chemistry of Copper
Cu2+ + e- → Cu+ E=0.15V
Cu+ + e- → Cu E=0.52V
Cu2+ + 2e- → Cu E=0.34V
By consideration of this data, it will be seen that any oxidant
strong enough to covert Cu to Cu+ is more than strong enough to
convert Cu+ to Cu2+ (0.52 cf. 0.14V). It is not
expected therefore that any stable Cu+ salts will exist in
aqueous solution.
Disproportionation can also occur:
2Cu+ → Cu2+ + Cu E=0.37V or
K=106
Coordination complexes
The reaction of EDTA4- with copper(II) gave a complex
where the EDTA was found to be pentadentate NOT hexadentate, unlike
other M(II) ions.
Cu(EDTA)2-
The structure of the [Cu(ox)2]2- ion can be described
as square planar or as a distorted octahedron when the packing in the
crystal lattice is considered. In the case of the sodium salt,
the individual units are parallel in the cell with the copper
linked to the oxygens coordinated to the copper in the
units sitting both above and below, whereas in the
potassium salt, the units are not parallel and when looking at three
units the central one is almost at right angles to the other two.
Here the copper is linked to one of the non-coordinated oxygens
in the units above and below it.
Na+ and K+ salts of [Cu(ox)2]2-
Cu(OH)2 reacts with NH3 to give a solution
which will dissolve cellulose. This is exploited in the
industrial preparation of Rayon. The solutions contain
tetrammines and pentammines. With pyridine, only tetramines are
formed eg Cu(py)4SO4.
The reaction of copper(II) with amino-acids has been extensively studied.
In nearly all cases the product contains the groups in a trans
configuration, which is expected to be the more stable. In the case
of glycine, the first product precipitated is always the cis- isomer
which converts to the trans- on heating.
See the
Laboratory Manual for C31L
for more details.
Analytical Determination of Copper(II)
A useful reagent for the analytical determination of the
copper(II) ion is the sodium salt of N,N-diethyldithiocarbamate.
In dilute alcohol solutions the presence of trace levels of
Cu2+ is indicated by a yellow colour, which can be
measured by a spectrophotometer, and the concentration determined
from a Beer's Law plot. The complex is
Cu(Et2dtc)2, which can be isolated as a
brown solid.
Cu(Et2dtc)2
References:
"Complexes and First-Row Transition Elements", D. Nicholls
"Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L. Gaus
"Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann
"Chemistry of the Elements", Greenwood and Earnshaw
return to the CHEM2101 (C21J) course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2000-2020 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire
(with grateful assistance from Cliff Riley and Jodi-Ann
Swaby),
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created June 2000. Links checked and/or last
modified 28th September 2020.
URL
http://wwwchem.uwimona.edu.jm/courses/copper.html


 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page