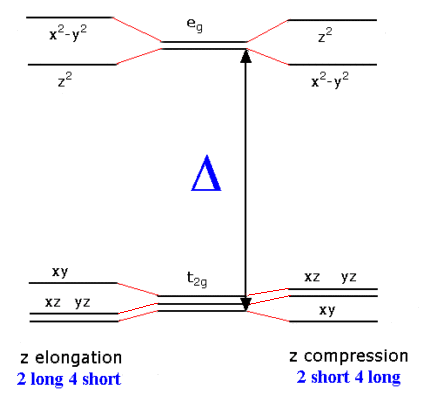
"any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy"In an octahedral crystal field, the t2g orbitals occur at lower energy than the eg orbitals. This is a reflection of the orientation of the orbitals since the t2g are directed between bond axes while the eg point along bond axes. The shielding effect this has on the electrons is used to explain why the Jahn-Teller effect is generally only important for odd number occupancy of the eg level.

| CuBr 2 | 4 Br at 240pm 2 Br at 318pm |
| CuCl 2 | 4 Cl at 230pm 2 Cl at 295pm |
| CuCl 2.2H 2O | 2 O at 193pm 2 Cl at 228pm 2 Cl at 295pm |
| CsCuCl 3 | 4 Cl at 230pm 2 Cl at 265pm |
| CuF 2 | 4 F at 193pm 2 F at 227pm |
| CuSO 4.4NH 3.H 2O | 4 N at 205pm 1 O at 259pm 1 O at 337pm |
| K 2CuF 4 | 4 F at 191pm 2 F at 237pm |
| KCuAlF 6 | 2 F at 188pm 4 F at 220pm |
| CrF 2 | 4 F at 200pm 2 F at 243pm |
| KCrF 3 | 4 F at 214pm 2 F at 200pm |
| MnF 3 | 2 F at 209pm 2 F at 191pm 2 F at 179pm |
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2000-2014 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,