Cobalt Chemistry
History
The
origin of the name Cobalt
is thought to stem from the German kobold for "evil spirits or goblins",
who were superstitiously thought to cause trouble for miners, since the cobalt
minerals contained arsenic that injured their health and the cobalt ores did not
yield metals when treated using the normal methods. The name could also be
derived from the Greek kobalos for "mine".
Cobalt was discovered in 1735 by the Swedish chemist Georg Brandt.
An excellent site for finding the properties of the elements,
including cobalt is at 
Further information on Cobalt can be found at the
Cobalt Institute Ltd
and
the page on Cobalt at Wikipedia.
Occurrence
The principal ores of Cobalt are cobaltite, [(Co,Fe)AsS], erythrite,
[Co3(AsO4)2.8(H2O)],
glaucodot, [(Co,Fe)AsS], and
skutterudite, [CoAs3].
World production of cobalt has steadily increased in recent years,
almost trebling since 1993. The dominance of African copper-cobalt producers
has been replaced by a more even spread of output between leading
producing countries, with Canada, Norway and more recently Australia,
together with exports from Russia, replacing lost production in the
Democratic Republic of Congo (Zaire). The strongest growth in production of cobalt has
come from Finland, where output grew at over 16% between 1990 and 2002.
An article on
The High Human Cost of Cobalt Mining may be of interest.
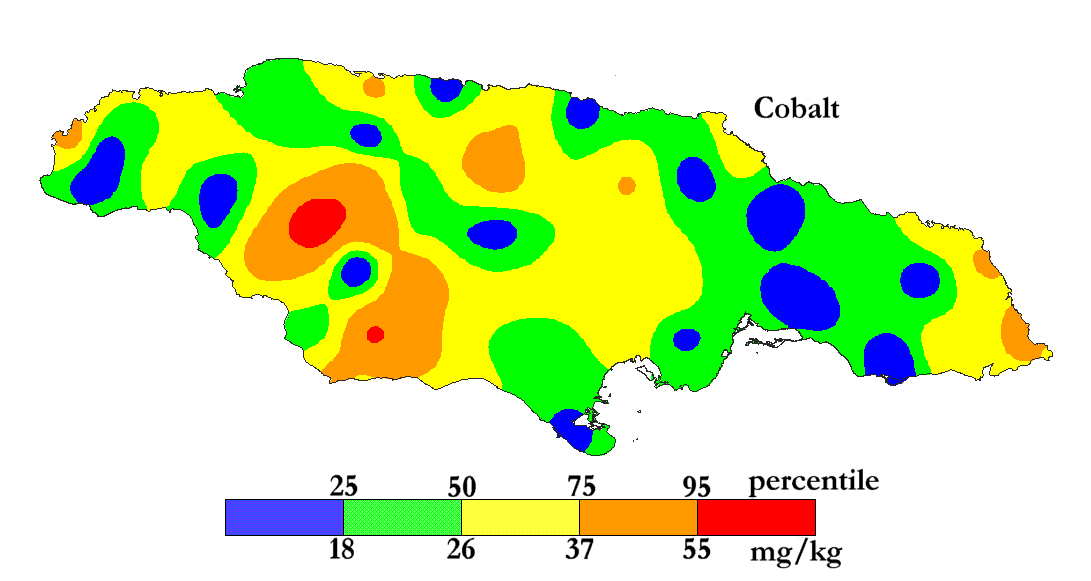
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Cobalt are shown below (courtesy of Prof G.C. Lalor).
Extraction
Not covered in this course.
Uses
- Alloys, such as:
Superalloys, for parts in gas turbine aircraft engines.
Corrosion- and wear-resistant alloys. Estimated as about 20% of production in 2003
- High-speed steels.
- Cemented carbides (also called hard metals) and diamond tools.
- Magnets and magnetic recording media.
- Catalysts for the petroleum and chemical industries.
- electroplating because of its appearance, hardness, and resistance to oxidation.
- Drying agents for paints, varnishes, and inks.
- Ground coats for porcelain enamels.
- Pigments (cobalt blue, known in ancient times, and Cobalt green).
- Battery sector (e.g. electrodes) estimated as about 11% of production in 2003.
- Steel-belted radial tires.
- Cobalt-60 has multiple uses as a gamma ray source:
* It is used in radiotherapy.
* It is used in radiation treatment of foods for sterilization (cold pasteurization).
* It is used in industrial radiography to detect structural flaws in metal parts.
Cobalt compounds
Oxides
Cobalt oxides
| Formula |
Colour |
Oxidation State |
MP |
Structure / comments |
| Co2O3 |
|
Co3+ |
|
|
| Co3O4 |
black |
Co2+/3+ |
900-950decomp |
normal spinel |
| CoO |
olive green |
Co2+ |
1795 |
NaCl -antiferromag. < 289 K |
Preparations:
Co2O3 is formed from oxidation of
Co(OH)2.
CoO when heated at 600-700°C converts to
Co3O4
Co3O4 when heated at 900-950°C reconverts
back to CoO.
Co3+ + e- ⇔ Co2+ 1.81V
Co2+ + 2e- ⇔ Co -0.28V
no stable [Co(H2O)6]3+ or
[Co(OH)3 exist since these convert to CoO(OH).
[Co(H2O)6]2+ not acidic and a stable carbonate
exists.
Cobalt Blue
One of the earliest uses of Cobalt was in the colouring of glass by the
addition of cobalt salts.
The pigment is based on the spinel CoAl2O4
and in the laboratory can be readily synthesised by pyrolysis of a
mixture of AlCl3 and CoCl2.
Halides
Cobalt(II) halides
| Formula |
Colour |
MP |
μ(BM) |
Structure |
| CoF2 |
pink |
1200 |
- |
rutile |
| CoCl2 |
blue |
724 |
5.47 |
CdCl2 |
| CoBr2 |
green |
678 |
- |
CdI2 |
| CoI2 |
blue-black |
515 |
- |
CdI2 |
Preparations:
Co or CoCO3 + HX → CoX2.aq →
CoX2
Cobalt complexes
The Cobalt(III) ion forms many stable complexes, which being
inert, are capable of exhibiting various types of isomerism. The
preparation and characterisation of many of these complexes dates
back to the pioneering work of
Werner
and his students.
Coordination theory was developed on the basis of studies
of complexes of the type:
Werner Complexes
| [Co(NH3)6]Cl3 |
yellow |
| [CoCl(NH3)5]Cl2 |
red |
|
trans-[CoCl2(NH3)4]Cl |
green |
|
cis-[CoCl2(NH3)4]Cl |
purple |
Another important complex in the history of coordination
chemistry is hexol.
This was the first complex that could be resolved into its optical isomers
that did not contain carbon atoms. Since then, only three or four others have been found.
Recently a structure that Werner apparently misassigned has been determined to be related
to the original hexol although in this case the complex contains 6 Co atoms,
i.e. is hexanuclear. The dark green compound is not resolvable into optical
isomers.
Werner's hexol and "2nd hexol"
A noticeable difference between chromium(III) and cobalt(III)
chemistry is that cobalt complexes are much less susceptible to
hydrolysis, though limited hydrolysis, leading to polynuclear
cobaltammines with bridging OH- groups, is well known.
Other commonly occurring bridging groups are
NH2-, NH2- and
NO2-, which give rise to complexes such as the
bright-blue amide bridged
[(NH3)5Co-NH2-Co(NH3)
5]5+.
In the preparation of cobalt(III) hexaammine salts by the oxidation
in air of cobalt(II) in aqueous ammonia it is possible to
isolate blue
[(NH3)5Co-O2-Co(NH3)
5]4+. This is moderately stable in concentrated
aqueous ammonia and in the solid state but readily decomposes in acid
solutions to Co(II) and O2, while oxidizing agents such as
(S2O8)2- convert it to the
green, paramagnetic
[(NH3)5Co-O2-Co(NH3)
5]5+ (μ300 = 1.7 B.M.).
In the brown compound both cobalt atoms are Co(III) and are joined by a
peroxo group, O22-, this fits with the
observed diamagnetism; in addition the stereochemistry of the
central Co-O-O-Co group is similar to that of
H2O2.
The green compound is less straightforward. Werner thought that it too
involved a peroxo group but in this instance bridging between Co(III) and Co(IV)
atoms.
This could account for the paramagnetism, but EPR evidence shows
that the 2 cobalt atoms are equivalent, and X-ray
evidence shows the central Co-O-O-Co group to be planar with an O-O distance
of 131 pm, which is very close to the 128 pm of the
superoxide, O2-, ion.
A more satisfactory formulation therefore is that of 2 Co(III) atoms
joined by a superoxide bridge.
A range of Co(II) dioxygen complexes are known, some of which are able
to reversibly bind O2 from the air. During WWII, some US aircraft
carriers are reported to have used these complexes as a solid source
for oxy-acetylene welding. By slightly warming the solid complex
the oxygen was released and when cooled again oxygen would be
coordinated again. Unlike an oxygen cylinder the solid would not
explode if hit by a stray bullet!
A
laboratory experiment designed to measure the uptake
of dioxygen by Cosalen is available online.
Co(acac)3 is a green octahedral complex of Co(III). In the case
of Co(II) a comparison can be made to the Ni(II) complexes.
Ni(acac)2 is only found to be monomeric at
temperatures around 200C in non-coordinating solvents such as
n-decane. 6-coordinate monomeric species are formed at room
temperature in solvents such as pyridine, but in the solid state
Ni(acac)2 is a trimer, where each Ni atom is
6-coordinate. Note that Co(acac)2 actually exists as a
tetramer.
|
[Ni(acac)2]3
|
[Co(acac)2]4
|
Cobalt(II) halide complexes with pyridine show structural isomerism.
Addition of pyridine to cobalt(II) chloride in ethanol can produce
blue, purple or pink complexes each having the composition
"CoCl2pyr2". The structures are 4, 5 and 6 coordinate with
either no bridging chlorides or mono- or di- bridged chlorides.
|
blue-[CoCl2pyr2] CN=4
|
pink-[CoCl2pyr2] CN=6
|
See the notes on isomerism
for examples of Co(III) compounds that show linkage and structural isomerism.
Health
see the notes at The University of Bristol on
Vitamin B12 and other Cobalt species essential
for good health.
return to the CHEM2101 (C21J) course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2002-2020 by Robert John Lancashire, all
rights reserved.
Created and maintained by Prof. Robert J.
Lancashire
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created July 2002. Links checked and/or last
modified 28th September 2020.
URL
http://wwwchem.uwimona.edu.jm/courses/cobalt.html


 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page