Chromium Chemistry
For the chapter on Chromium chemistry from the Elsevier text
"Chemistry of the Elements" by Greenwood and Earnshaw see
On-Line Metals Based Surveys
History
Discovered in 1797 by the French chemist Louis Nicolas Vauquelin,
it was named chromium (Greek chroma, "colour") because of the
many different colours characteristic of its compounds.
Occurrence
See the
International Chromium Development Association web site
for some background material, especially the article on
chromium in the environment.
Chromium is the earth's 21st most abundant element
(about 122 ppm) and the 6th most abundant transition
metal.
The principal and commercially viable ore is chromite,
FeCr2O4, which is found mainly in southern Africa
(with 96% of the worlds reserves), the former U.S.S.R and the
Philippines. Less common sources include crocoite,
PbCrO4, and chrome ochre, Cr2O3,
while the gemstones emerald and ruby owe their colours to traces
of chromium.
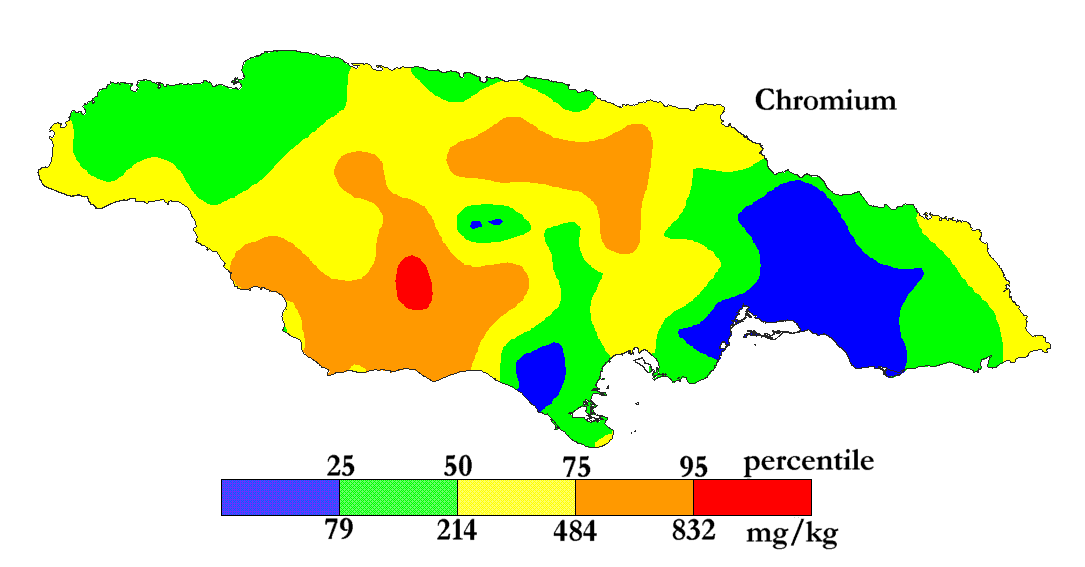
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Chromium are shown below (courtesy of Prof G.C. Lalor).
Extraction
Chromite, FeCr2O4, is the most commercially useful
ore, and is extensively used for extraction of chromium. Chromium
is produced in two forms: (Chemistry of the Elements, Greeenwood
and Earnshaw, Chapter 23).
(a) Ferrochrome by the reduction of chromite with coke in an
electric arc furnace. A low-carbon ferrochrome can be produced by
using ferrosilicon instead of coke as the reductant. This
iron/chromium alloy is used directly as an additive to produce
chromium-steels which are "stainless" and hard.
(b) Chromium metal by the reduction of
Cr2O3. This is obtained by aerial oxidation
of chromite in molten alkali to give sodium chromate,
Na2CrO4, which is leached out with water,
precipitated and then reduced to the Cr(III) oxide by carbon. The
oxide can be reduced by aluminium (aluminothermic process) or
silicon:
Cr2O3 + 2Al → 2Cr +
Al2O3
2Cr2O3 + 3Si → 4Cr + 3SiO2
The main use of the chromium metal so produced is in the
production of nonferrous alloys, the use of pure chromium being
limited because of its low ductility at ordinary temperatures.
Alternatively, the Cr2O3 can be dissolved
in sulphuric acid to give the electrolyte used to produce the
ubiquitous chromium-plating which is at once both protective and
decorative. The sodium chromate produced in the isolation of
chromium is itself the basis for the manufacture of all
industrially important chromium chemicals. World production of
chromite ores approached 12 million tonnes in 1995.
Properties
An excellent site for finding the properties of the elements,
including chromium is at 
For an article that reviews simple chemistry see
chemguide.
Chromium Compounds
Most compounds of chromium are coloured (why is Cr(CO)6 white?);
the most important are the chromates and dichromates of sodium and potassium
and the potassium and ammonium chrome alums.
The dichromates are used as oxidizing agents in quantitative
analysis, also in tanning leather. Other compounds are of
industrial value; lead chromate is chrome yellow, a valued
pigment. Chromium compounds are used in the textile industry as
mordants, and by the aircraft and other industries for anodizing
aluminium.
Halides
Chromium(III) halides
| Formula |
Colour |
MP |
M-X (pm) |
μ(BM) (b) |
Structure |
| CrF3 |
green |
1404 |
190 |
- |
- |
| CrCl3 |
red-violet |
1152 |
238 |
- |
CrCl3 |
| CrBr3 |
green-black |
1130 |
257 |
- |
BiI3 |
| CrI3 |
black |
>500decomp |
- |
- |
- |
(b) all 3.7-4.1 BM.
Preparations:
CrX3 are prepared from Cr with X2,
dehydration of CrCl3.6H2O requires
SOCl2 at 650C.
Chromium(II) halides
| Formula |
Colour |
MP |
μ (BM) |
Structure |
| CrF2 |
green |
894 |
4.3 |
distorted rutile |
| CrCl2 |
white |
820-824 |
5.13 |
distorted rutile |
| CrBr2 |
white |
844 |
- |
- |
| CrI2 |
red-brown |
868 |
- |
- |
Preparations:
Reduction of CrX3 with H2/HX gives
CrX2.
Oxides
Chromium oxides
| Formula |
Colour |
Oxidation State |
MP |
Magnetic Moment |
| CrO3 |
deep red |
Cr6+ |
197decomp |
- |
| Cr3O8 |
- |
intermediate |
- |
- |
| Cr2O5 |
- |
- |
- |
- |
| Cr5O12 etc |
- |
- |
- |
- |
| CrO2 |
brown-black |
Cr4+ |
300decomp |
- |
| Cr2O3 |
green |
Cr3+ |
2437 |
-antiferromagnetic < 35 C |
Dichromate and chromate equilibria is pH dependent:
HCrO4- →
CrO42- + H+ K=10-5.9
H2CrO4 → HCrO4- +
H+ K=10+0.26
Cr2O72- + H2O →
2HCrO4- K=10-2.2
HCr2O7- →
Cr2O72- + H+
K=10+0.85
Hence the variation found for solutions of CrO3 are:
pH > 8 CrO42- yellow
pH 2-6 HCrO4- and
Cr2O72- orange-red
pH < 1 H2Cr2O7
One of the most obvious characteristics of Cr(III) is that it is
acidic i.e it has a tendency to hydrolyse and form polynuclear
complexes containing OH- bridges in a process known as
OLATION.
This is thought to occur by the loss of a proton from coordinated
water, followed by coordination of the OH- to a second
cation:
[Cr(H2O)6]3+ →
[Cr(H2O)5(OH)]2+ →
H
O
/ \
[(H2O)4Cr Cr(H2O)4]4+ pK=4 etc.
\ /
O
H
The ease with which the proton is removed can be judged by the
fact that the hexaaquo ion (pKa ~ 4) is almost as strong as
acetic acid. Further deprotonation and polymerization can occur
and, as the pH is raised, the final product is hydrated
chromium(III) oxide or "chromic hydroxide".
Representative Complexes
The Chromium(III) ion forms many stable complexes and since
they are inert are capable of exhibiting various types of
isomerism.
![anhydrous CrCl3 and hydrated trans-[CrCl2(H2O)4]Cl.2H2O](../gifs/crcl3.jpg)
anhydrous CrCl
3 and hydrated "CrCl
3.6H
2O",
Hydrated chromium chloride, "CrCl3.6H2O", exists as hydrate
isomers, including:
the violet [Cr(H2O)6]Cl3
the dark green trans-[CrCl2(H2O)4]Cl.2H2O
salt shown above, etc.
the pale green [CrCl(H2O)5]Cl2.H2O
Anhydrous CrCl3 reacts with pyridine only in
the presence of Zinc powder. This allows a small amount of the
Cr(II) ion to be formed, which is very labile but unstable with respect
to oxidation back to Cr(III).
CrCl3 + pyr/Zn → CrCl3pyr3
See the laboratory
manual for this course for a range of other Cr(III) complexes for which you
should know the structure.
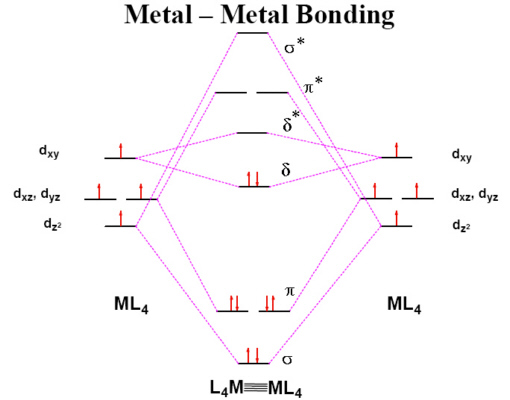
[Cr2(OAc)4].2H2O is an
example of a Cr(II) complex which is reasonably stable in air
once isolated. Each Cr(II) ion has 4 d electrons but the complex
is found to be diamagnetic which is explained by the formation of
a quadruple bond between the two metal ions. The Cr-Cr bond
distance in a range of these quadruply bonded species has been
found to vary between 195-255 pm.
In case you think that quadruple bonds are as far as it goes....
A recent report describes the structure of a Cr complex with a
quintuple bond between two Cr(I) ions.
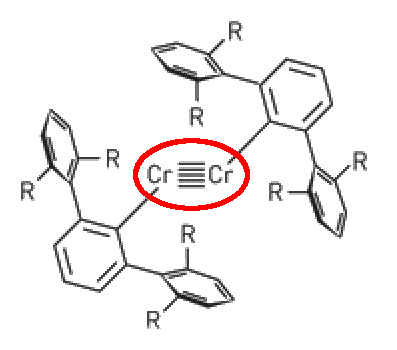
Cr(I) - Cr(I) quintuple bonded structure.
The compound Ar'CrCrAr' (R = isopropyl) was very air and moisture sensitive and crystallised as
dark red crystals. X-ray diffraction revealed a
Cr-Cr bond length of about 184 pm and a planar, trans-bent core geometry.
Published in Science by P Power et. al., UCLA Davis, 22 September 2005
[DOI: 10.1126/science.1116789].
Another recent innovation is the formation of "zeolite-type" architectures from
Metal-Organic-Frameworks (MOF's). The synthesis of MIL-101 consists of the
hydrothermal reaction of 1,4-benzene dicarboxylate, H2BDC
(166 mg, 1 mmol) with
Cr(NO3)3.9H2O (400 mg, 1 mmol),
hydrofluoric acid (1 mmol), and 4.8 mL of H2O (265 mmol)
for 8 h at 220 °C, producing a pure and highly crystallized
green powder of the chromium terephthalate with formula
Cr3F(H2O)2O[(O2C)-C6H4-(CO2)]3.nH2O (n=25), based
on chemical analysis.
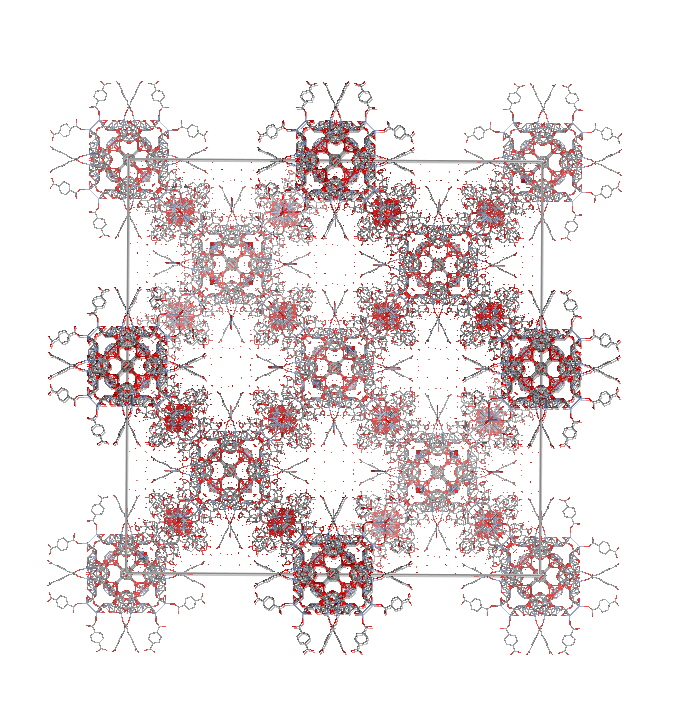
MIL 101 Chromium MOF structure.
"First Direct Imaging of Giant Pores of the Metal-Organic Framework MIL-101"
Millange and co-workers
Chem. Mater. 2005, 17, 6525-6527
Uses
More than half the production of chromium goes into metallic
products, and about another third is used in refractories. It is
an ingredient in several important catalysts. The chief use of
chromium is to form alloys with iron, nickel, or cobalt. The
addition of chromium imparts hardness, strength, and corrosion
resistance to the alloy. In the stainless steels, chromium makes
up 10 percent or more of the final composition. Because of its
hardness, an alloy of chromium, cobalt, and tungsten is used for
high-speed metal-cutting tools. When deposited electrolytically,
chromium provides a hard, corrosion-resistant, lustrous finish.
For this reason it is widely used as body trim on automobiles and
other vehicles. The extensive use of chromite as a refractory is
based on its high melting point, its moderate thermal expansion,
and the stability of its crystalline structure.
In chromites and chromic salts, chromium has a valence of +3.
Most of these compounds are green, but some are red or blue.
Chromic oxide (Cr2O3) is a green solid. In
chromates and dichromates, chromium has a valence of +6.
Potassium dichromate (K2Cr2O7)
is a red, water-soluble solid that, mixed with gelatin, gives a
light-sensitive surface useful in photographic processes. The
chromates are generally yellow, the best known being lead
chromate (PbCrO4), an insoluble solid widely used as a
pigment called chrome yellow. Chrome green is a mixture of chrome
yellow and Prussian blue.
Chromium is used to harden steel, to manufacture stainless steel,
and to form many useful alloys. Much is used in plating to
produce a hard, beautiful surface and to prevent corrosion.
Chromium gives glass an emerald green colour and is widely used
as a catalyst. The refractory industry has found chromite useful
for forming bricks and shapes, as it has a high melting point,
moderate thermal expansion, and stability of crystalline
structure.
Health
Chromium is an essential trace element in mammalian metabolism.
In addition to insulin, it is responsible for reducing blood
glucose levels, and is used to control certain cases of diabetes.
It has also been found to reduce blood cholesterol levels by
diminishing the concentration of (bad) low density lipoproteins
"LDLs" in the blood. It is supplied in a variety of foods such as
Brewer's yeast, liver, cheese, whole grain breads and cereals,
and broccoli. It is claimed to aid in muscle development, and as
such dietary supplements containing chromium picolinate (its most
soluble form), is very popular with body builders.
|
mer- isomer of Cr(III) picolinate complex.
|
Ammonium Reineckate,
NH4(Cr(NH3)2(SCN)4).H
2O, is used to test for the presence of dihydromorphinone
and other substances generally found in persons involved in substance
abuse.
References:
"Inorganic Chemistry", 3rd Edition, Catherine Housecroft, Alan G. Sharpe, Publisher: Prentice Hall
"Complexes and First-Row Transition Elements", D. Nicholls
"Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L. Gaus
"Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann
"Chemistry of the Elements", Greenwood and Earnshaw
"Hydrolysis of Cations", Baes and Messmer
return to the CHEM2101 (C21J) course outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2002-2020 by Robert John Lancashire,
all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire
(with grateful assistance from Llorenia Muir-Green),
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created July 2002. Links checked and/or last
modified 28th September 2020.
URL
http://wwwchem.uwimona.edu.jm/courses/chromium.html

![anhydrous CrCl3 and hydrated trans-[CrCl2(H2O)4]Cl.2H2O](../gifs/crcl3.jpg)


 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page