Inorganic Laboratory Experiments
Aims and Objectives
These experiments were designed to be carried for CHEM2101 and some are
included in the CHEM2111 Laboratory course of 4 hour sessions.
In the old course:
In the first week, several simple coordination complexes are
prepared.
For weeks 2,3 and 4 a number of spectroscopic investigations are
performed, including: IR, UV/Vis and a determination of a
magnetic moment. This is done using a rotation scheme to
accommodate the time required at each instrument and to give each
student the opportunity to record their own spectra. A part of
the session will be spent in the Chemistry Resource Centre using
the Tanabe-Sugano programs to interpret the Visible spectra
recorded.
During this period as well, some qualitative tests are
performed on first row transition metal ions and the analysis of the
ferrioxalate complex is performed.
In the fifth week, a kinetics experiment on the acid hydrolysis
of a metal complex is carried out as a group exercise. Each
student is assigned a variation such as initial concentration of
complex, temperature, ionic strength or [H+] concentration.
At the end of the session, all rate constants are pooled so that
more extensive analysis can be done.
In the final week, any outstanding experiments are completed.
As you do these experiments you should work toward the following
objectives:
- Improve and extend your laboratory skills in handling solids,
handling liquids, carrying out stoichiometric calculations, and
performing standard laboratory procedures like weighing,
qualitative and quantitative liquid transfer, reflux, gravity and
suction filtration, recrystallization, volume reduction, and the
determination of melting point/decomposition temperature.
- Acquire skills in sample preparation for and acquisition of
IR spectra, UV-visible spectra, and magnetic susceptibility
measurements.
- Acquire and/or improve skills in planning for an effective
laboratory session.
- Improve and extend your laboratory record-keeping
skills.
- Acquire and improve skills in the strategy of chemical
synthesis for relatively simple chemical systems.
- Acquire skills in interpretation of characterizational
data
- Acquire and/or improve formal writing skills.
- Enhance your ability to work independently in the
laboratory.
- Enhance your confidence in your ability to work in the
laboratory.
- Improve your speed and efficiency in the laboratory.
- Improve your ability to be self-critical (that is, to notice
inconsistencies or abnormalities in your experimental findings
and to take steps to check their reliability).
It is intended that the laboratory experiments will reinforce
the lecture material and give students practice at assigning
spectral bands and interpreting magnetic properties as well as
giving an introduction to the study of reaction kinetics.
Experiment 2016-1 Perborate synthesis
Experiment 2016-2 Computer Laboratory on Symmetry elements and operations
Experiment 1:
Preparation of some chromium(III) complexes, either:
1) [Cr(en)3]Cl3
and K[CrEDTA(H2O)]
2) [Cr(urea)6]Cl3
and K3[Cr(NCS)6]
or 3) cis-[CrCl2(en)2]Cl.1.5 H2O
and cis-K[Cr(ox)2(H2O)2].2H2O
The final reports should include the Vis and IR spectroscopic
analysis and Gouy or Evans Method
determination of the magnetic moment. A simulation of
the Gouy Method is another option. It is expected that the
discussions will be based on the appropriate Tanabe-Sugano diagrams and
include an assessment of how the measured values compared with
what was expected.
Experiment 2:
Preparation and analysis of potassium trisoxalatoiron(III)
trihydrate
Experiment 3a:
Preparation and aquation of
trans-dichlorobis(1,2-diaminoethane)cobalt(III) chloride
Experiment 3b:
Preparation of pentaamminechlorocobalt(III) chloride and the
kinetics of its aquation
Experiment 4:
Preparation of some Werner
complexes.
Experiment 5:
Preparation and reactions of ferrocene.
Experiment 6:
Qualitative Tests: Each student should carry out the
qualitative tests on 2 transition metal ions.
All samples should be submitted in properly labelled (name,
sample, weight etc.) containers for marking, together with your
reports.
Experiment 1.
The preparation of some chromium(III) complexes
1 a) Preparation of
tris-(1,2-diaminoethane)chromium(III) chloride,
[Cr(en)3]Cl3
1 g granular zinc, 2.66 g CrCl3.6H2O, 10
cm3 of 1,2-diaminoethane (ethylenediamine), and 10
cm3 of methanol are refluxed on a steam bath for one
hour.
The solution is then cooled to room temperature and the yellow
product collected on a sintered glass. The filtered product is
then washed with acetone/methanol mixture until the washings are
colourless. Unreacted zinc is separated by dissolving the product
using a minimal amount of distilled water. The yellow solid is
precipitated from the filtrate with acetone. Filter and allow the
product to dry, determine the percentage yield and transfer to a
labeled vial.
The X-ray structure of this
complex has been determined and can be viewed with Jmol.
1 b) Preparation of
K[Cr(EDTA)(H2O)]
A suspension of H4EDTA (3 g) and KOH (0.8 g) in 25
cm3 of distilled water is transferred to a Teflon
reactor. To this suspension is added
Cr2(SO4)3 (3.3 g). The reactor
is securely sealed and heated in a microwave oven for 5 minutes.
The resulting red solution is transferred to an evaporating dish
and placed on a steam bath. Evaporate until dryness, and collect
the purple solid using methanol as washing solution. Dry at the
pump and determine the yield.
The X-ray structure of this
complex has been determined and can be viewed with Jmol.
Note that the EDTA has one "arm dangling".
2 a) Preparation of
hexakis-(urea)chromium(III) chloride
Chromium chloride hydrate (2.7 g) and urea (3.6 g) are dissolved
in 10 cm3 of distilled water and a few drops of 3M HCl
is added. The solution is heated on a steam bath until a
crystalline crust forms. The slurry obtained is dissolved in the
minimum of water at 50-60 C and rapidly filtered. The salt
crystallizes as light green needles. Dry at the pump and
determine the yield.
The X-ray structure of this
complex has been determined and can be viewed with Jmol.
The FTIR of this
complex has been determined and can be viewed with JSpecView.
2 b) Preparation of Potassium
hexathiocyanatochromate(III),
K3[Cr(NCS)6]
Make an aqueous solution of potassium thiocyanate, KSCN (2.5 g), chrome alum
( {KCr(SO4)2.12H2O} 3.0 g)
using distilled water (10 cm3). Pour the solution into
an evaporating dish and place on a steam bath. Evaporate to
dryness, to obtain a mass of red crystals. Extract the solid, via
suction filtration, using alcohol. The desired
K3[Cr(NCS)6], should dissolve very readily
while K2SO4 remains as a residue. After
evaporation of the filtered alcohol extract, collect the dark
red-violet crystals. Dry at the pump and determine the
yield.
The X-ray structure of this
complex has been determined and can be viewed with Jmol.
3 a) Preparation of
cis-dichlorobis-(1,2-diaminoethane)chromium(III) chloride,
cis-[CrCl2(en)2]Cl.1.5H2O
In a 250 cm3 beaker, dissolve chromium chloride (10 g)
in 30 cm3 dimethylformamide. Heat the solution on a
hot plate and reduce the volume to half. Add 5 cm3
1,2-diaminoethane slowly using a dropper. Precipitate the purple
solid using methanol and collect using a sintered glass filter
funnel. Dry at the pump and determine the yield.
The X-ray structure of this
complex has been determined and can be viewed with Jmol.
3 b) Preparation of
cis-K[Cr(ox)2(H2O)2].2H2O.
Prepare an intimate mixture of finely ground potassium dichromate
(2 g) and oxalic acid dihydrate (6 g) and heap the powder in a 15
cm evaporating dish. Place one drop of water in a small
depression in the mixture and cover the dish with a watch glass.
After a short induction period the reaction commences and soon
becomes vigorous with the evolution of steam and carbon dioxide.
The product of this reaction is a purple viscous liquid over
which is poured 20 cm3 of ethanol and the mixture stirred and
ground with a glass rod until the product solidifies. If
solidification is slow, decant the liquid and repeat the process
with a second portion of ethanol. Filter, dry at the pump, and
record the yield.
The X-ray structure of this
complex has been determined and can be viewed with Jmol.
Experiment 2.
The preparation of Potassium tris(oxalato)ferrate(III) trihydrate
Mark the level of 45 cm3 water in a 250 cm3 beaker. To a
well-stirred solution of 5 g of ferrous ammonium sulfate in 20
cm3 of warm water containing 1 cm3 of dilute sulfuric acid in the
beaker, add a solution of 2.5 g of oxalic acid dihydrate in 25
cm3 of water. Slowly heat the mixture to boiling (beware of
bumping) then allow thc yellow precipitate to settle. Decant the
supernatant through a Buchner funnel making sure it has a
properly fitted filter paper. Add 15 cm3 of hot water to the
solid, stir and filter. Drain well and then transfer all the
precipitate from the paper back into the beaker with 10 cm3 hot
water.
Add 3.5 g solid potassium oxalate monohydrate and heat to
approximately 40 C. Add slowly, using a dropper, 9 cm3 of "20
vol" hydrogen peroxide. (If the precipitate looks yellowish, not
brown and settles readily, decant the supernatant, add a solution
of 0.2 - 0.4 g potassium oxalate monohydrate in 1 - 2 cm3 water
and then hydrogen peroxide dropwise until the precipitate
dissolves. Then add the previously decanted supernatant). Heat to
boiling, and add a solution of 2 g of oxalic acid dihydrate in 30
cm3 of water in portions, add 20 cm3 initially, then if the brown
precipitate still remains, add more solution little by little
until it all dissolves. Boil the clear solution down to a volume
of 40 - to 50 cm3, filter through a Buchner funnel with well
fitting paper and add 95% ethanol slowly until a precipitate
starts to form (~30 cm3). Redissolve any crystals by heating
(beware of fire) and leave to crystallise.
Filter and wash the crystals on the Buchner with a 1:1 ethanol /
water mixture and finally with acetone, (beware fire
again). Dry in the air and weigh. The complex is
photosensitive and should not be exposed to light unnecessarily.
Store in a sample bottle wrapped in foil.
An IR spectrum is
available.
Determination of the oxalate content of Potassium
trisoxalatoferrate(III) trihydrate.
The iron(III) complex is first decomposed in hot acid solution
and the free oxalic acid is titrated against standard (0.02 M)
potassium permanganate solution. No indicator is required.
In duplicate, weigh accurately about 0.2 g of the potassium
trisoxalatoferrate(III) complex previously prepared. Boil the
sample with 50 cm3 of 1 M sulfuric acid in a conical
flask. Allow the solution to cool to about 60°C and titrate
slowly with the potassium permanganate solution provided (which
you will need to standardise). Continue until the warm solution
retains a slight pink colouration after standing for about 30
sec.
Calculate the percentage by weight of oxalate in the complex,
compare this with the theoretical value and thus obtain the
percentage purity of the complex.
MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
C2O42- → 2CO2 + 2e-
Photochemical reactions of Potassium trisoxalatoferrate(III)
trihydrate.
Prepare duplicate solutions containing 0.2 g accurately weighed
of your sample in 15 cm3 of dilute sulfuric acid. Dilute the
solutions to 50 cm3 with distilled water and expose
them to sunlight for one hour (note carefully what happens).
Titrate with your standardised permanganate to determine the
amount of reducing agent present.
Expose a small portion of your product to sunlight for several
hours. Make sure that the crystals have been ground to a fine
powder and that you periodically stir the crystals so that all
the sample gets exposed equally to the sunlight. Perform the
following tests on samples of both irradiated and unirradiated
complex:
Dissolve your sample in dilute sulfuric acid and divide the
solution into three.
1) treat with a freshly prepared solution of potassium ferrocyanide.
2) treat with a freshly prepared solution of potassium ferricyanide.
3) treat with a solution of potassium thiocyanate.
Record carefully all observations.
Experiment 3:
The Mechanism of Aquation of
trans-dichlorobis(1,2-diaminoethane)cobalt(III)
chloride
Introduction
Coordination complexes of cobalt(III) undergo ligand exchange or
substitutions slowly as compared to many other transition metal
compounds. Their slow reactions have made them suitable for
kinetic investigations of their reaction mechanisms. The present
experiment involves a kinetic study of the acid hydrolysis of
trans-[CoCl2(en)2]Cl, whereby the
probable mechanism of the octahedral cobalt(III) substitution can
be determined. The reaction will be conducted such that each
student will investigate one of the following variations: pH,
temperature, concentration and ionic strength. The entire class
will collate these results which can be used to show the effect
of these variations on the aquation.
Complexes of this type undergo aquation in a step-wise fashion
according to the equations:
trans-[CoCl2(en)2]+ + H2O → trans-[CoCl(en)2(H2O)]2+ + Cl-
trans-[CoCl(en)2(H2O)]2+ + H2O → [Co(en)2(H2O)2]3+ + Cl-
where the first step is the one to be measured quantitatively in
this experiment.
In principle, two fundamentally different mechanisms are
possible for these reactions; a dissociative or associative
mechanism. The kinetic rate laws expeected for these two types of
mechanism are:
dissociative: Rate = k1[complex]
associative: Rate = k2[complex][H2O]
= kobs[complex]
that is they are dependent only on the concentration of the
complex and are first order. This observation, however, furnishes
no information as to the role played by the water and does not
give any information about the molecularity of these
reactions.
Nevertheless the way in which the rate constant is affected by
various changes in the nature of the complex ion is expected to
give us information about the mechanism. It has been found that
increasing chelation such as replacing two NH3 ligands
by one ethylenediamine slows down the rate of acid hydrolysis.
Allowing for the chelation effect the divalent monochloro
complexes react about 100 times slower than the univalent
dichloro complexes.
Preparation of
trans-[CoCl2(en)2]Cl
CoCl2,6H2O (2 g) is dissolved in 2 cm3 of
water in a beaker and 1 cm3 of 1,2-diaminoethane (en) in 5 cm3 of
water is slowly added cautiously and with stirring. The solution
is cooled in an ice bath to 5°C and 2 cm3 of H2O2 (30%) is slowly
added while maintaining the temperature at 5°C. [
CAUTION: Keep H2O2 off the skin and eyes!]. Then the
solution is gently warmed to about 60-70°C for 15-20
minutes.
Concentrated HCl (4 mL) is then added, and the solution
evaporated on a steam bath with occasional stirring to about 10
cm3. After cooling the solution in an ice-bath, 3 mL of ethanol
is added and the mixture cooled for a further 10 minutes. The
resultant green crystals of
trans-[CoCl2(en)2]Cl.HCl.2H2O
are filtered onto a sintered glass Buchner funnel, washed with
ethanol and sucked dry.
An IR spectrum is
available.
To drive off the HCl of crystallization, place the dark green
crystals in a small beaker containing 5 cm3 of methanol and
vigorously stir these with a glass stirring rod. Transfer the
resultant slurry to a large test tube. Place the test tube in a
beaker of water, then heat the water (gently at first) until the
methanol has evaporated and no more HCl gas is driven off. (The
presence of HCl can be tested by holding a piece of moist litmus
paper at the mouth of the test tube).
Boiling for 15 minutes after the methanol has evaporated is
usually sufficient. Trans-[CoCl2(en)2]Cl thus obtained is a light
green powder.
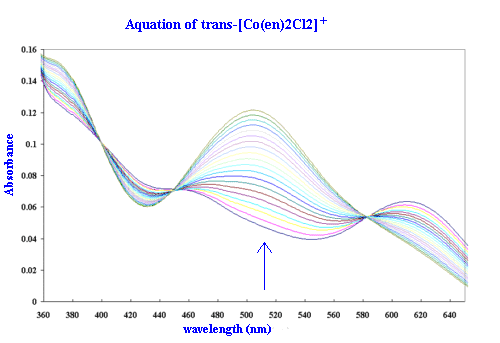
Spectral Studies
The visible spectrum of the complex ion, trans-[CoCl2(en)2]Cl,
has been investigated thoroughly. The peak at 625 nm is clearly
defined with an extinction coefficient of 3.33 m2mol-1. The trans
configuration is well characterized by its shoulder at 440 nm. In
acidic solution the trans-dichloro complex gradually converts to
a mixture of 35% cis- and 65% trans-[CoCl(en)2(H2O)]2+. By
repetitively scanning the spectrum, this conversion is revealed
by the presence of three poorly defined isosbestic points (588
nm, 448 nm and 408 nm).
Kinetic Studies
Kinetic runs for the acid hydrolysis of
the trans-[CoCl2(en)2]Cl complex are to be recorded (for
single beam use 515 nm where the increase in absorbance shows a
maximum change to occur between the reactant and product). In
addition to the above CSV file of the full spectrum run over
several hours, the first and
last scan from a typical
student run are available as JCAMP-DX files for comparison.
Each student will be assigned to study the effect of one
variation on the observed rate constants, from the following
parameters:
(a) Complex concentration (3 mM to 15 mM).
(b) Hydrogen ion concentration (0.1 M to 0.5 M).
(c) Ionic strength (0.1 to 0.5 M).
(d) Temperature (25 to 45°C).
A typical run should be done as follows.
Add required volumes of stock HNO3 and
NaNO3 solutions to a 50 cm3 volumetric
flask and add distilled water to make it about 80% full. The
flask is then immersed in a thermostatic water bath for
temperature equilibration. Weigh the required amount of the
complex on an analytical balance and dissolve the complex with a
small volume of distilled water in a beaker. Transfer the
solution quantitatively to the thermostated 50 cm3 flask and make
up to the mark with distilled water. Transfer immediately about 3
cm3 of the solution from the flask to a 1 cm glass or plastic
cell, start your stop watch and place the cell in the cell holder
of the spectrophotometer. Read and record the absorbance at 10
minute intervals for at least 2 hours. The absorbance reading at
infinite time can be taken after 5 hours. Alternatively the
reading at infinite time can be obtained by first warming a
sample of the mixture on a water-bath for 5 minutes and then
placing in the spectrometer.
For first order kinetics the following rate expression is
expected:
ln( (Ainf - At) / (Ainf -
A0) ) = kt
where k is the rate constant
A0 is the initial Absorbance
Ainf is the Absorbance at infinite time
and At is the Absorbance at any time, t.
Calculate the pseudo first order rate constants by plotting
ln(Ainf - At)vs time, t, in sec.
Questions
1. Draw the two structures of the cis and trans isomers of
[CoCl2(en)2]Cl. Which, if any, of these
geometric isomers is potentially resolvable into optically active
isomers?
2. The green product first isolated, is best represented as
trans-[CoCl2(en)2]+[H5O
2]+2Cl-. This contains an example of a hydrated
proton, where the O-H-O moiety is linear and the O-O separation
is 200 pm. Draw the likely structure of the cation. Give examples
of other known hydrated proton structures.
For a clue see here.
3. In the complex
[CoCl(NH3)5]2+ increasing
chelation such as replacing the two NH3 ligands by one
ethylenediamine slows down the rate of acid hydrolysis. Why?
4. Why should the rate of hydrolysis of
[CoCl2(en)2]+ be 100 times
faster than that of
[CoCl(en)2(H2O)]2+ ?
5 Tabulate all the experimental data collected during the class
and comment on the effect of (a) pH, (b) temperature and (c)
ionic strength on the rate of hydrolysis.
6. Calculate ΔH# and ΔS# values from your temperature dependence
data and compare these values with the values obtained for other
chloroamine complexes of cobalt(III).
| Complex |
k |
ΔH# (kJ/mol) |
ΔS# (J/deg mol) |
|
trans-[CoCl2([13]aneN)4]+ |
6.76 x 10-4 |
107.1 |
52.7 |
|
trans-[CoCl2([14]aneN4)]+ |
1.1 x 10-6 |
102.9 |
-12.6 |
| trans-[CoCl2(trien)]+ |
3.5 x 10-3 |
108.8 |
64.9 |
|
trans-[CoCl2(en)2]+ |
3.2 x 10-5 |
109.6 |
58.6 |
References
F. Basolo and R.G. Pearson, Mechanism of Inorganic Reactions,
2ed., John Wiley and Sons, New York, 1967, p162.
B. Douglas, D.H. Daniels and J.J. Alexander, Concepts and Models
of Inorganic Chemistry, 2nd Edition, John Wiley and Sons Inc.,
New York, 1983, pps 361-363.
Experiment 5 :
Preparation of Ferrocene
Introduction
The first step involves the preparation of cyclopentadiene. This
is obtained by cracking the dimer, dicyclopentadiene, by
distillation (BP ~42C). This corresponds to a retrograde
Diels-Alder reaction and the cyclopentadiene monomer thus
obtained dimerises slowly (t ½ ~12 hours at room
temperature) and should be used without delay. It is also highly
flammable and should be stored in ice.
The next step makes use of the fact that alkali metal hydroxides
are able to deprotonate the cyclopentadiene when used in a
non-hydroxylic solvent in which they are essentially insoluble,
in this case DMSO.
The final step is the reaction with the ferrous chloride which
should be done quickly to avoid too much air affecting the
reaction.
It is essential to efficiently coordinate these steps of the
procedure if good yields are to be obtained. The experiment MUST
be carried out in a fume hood.
Procedure
The equipment for cracking the dicyclopentadiene will be set up
by a demonstrator. Each student requires 4 cm3 of the
cyclopentadiene. Take two boiling tubes each fitted with a cork.
Into the first place 6 cm3 of dimethyl sulfoxide and 3g of KOH
pellets which have been finely ground in a mortar and pestle.
(Caution - If KOH is spilt on the bench, it needs to be cleaned
up immediately).
Into the second boiling tube, place 10 cm3 of dimethyl sulfoxide
and 2 g of anhydrous ferrous chloride.
The air in the boiling tubes must now be replaced by dinitrogen
and the tubes shaken. Consult the demonstrator for the use of the
dinitrogen gas cylinder.
The next two operations must be carried out quickly so as to
prevent too much air getting into the boiling tubes. Add the 4
cm3 of cyclopentadiene to the KOH suspension, stopper the tube
and shake well. When a dark red brown colour develops in the tube
(about 3 minutes) add the ferrous chloride solution, stopper and
shake. Cool in ice if it gets too hot. Allow the mixture to stand
for 5 minutes then pour it into 200 cm3 of cold water and filter
the resulting mixture using a Buchner Funnel. After drying at the
pump for 20 minutes, transfer the solid and filter paper to a dry
200 cm3 beaker. Add 50 cm3 of 60-80 petroleum ether and heat to
boiling on a water-bath. Filter into another dry 200 cm3 beaker
using a filter funnel fitted with fluted filter paper.
Concentrate the resulting orange solution on a water-bath until
crystallisation begins (reduce to 5-10 cm3). Cool in ice to
complete crystallisation.
After standing for 30 minutes, filter off the crystalline
product and dry at the pump. This last filtration may be carried
out in the open laboratory. Record your yield as a percentage
based on FeCl2 and determine the M.P (this should be
compared to the literature value).
Reactions of Ferrocene
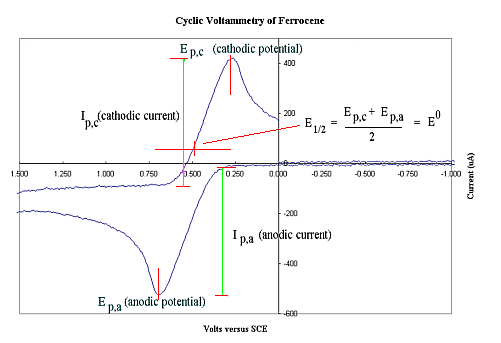
a) Oxidation
A cyclic voltammogram of ferrocene (0.1 mM)
recorded in DMF using [n-Bu4N]PF6 (0.1M)
as supporting electrolyte shows a reversible oxidation wave at
E1/2 ~0.5 Volt which indicates that at a potential
slightly higher than this (eg 0.6 V) a bulk electrolysis
experiment should produce the ferrocinium cation.
Alternatively an oxidant such as H2SO4 can
be used. The ferricinium ion
[Fe(C5H5)2]+ produced
is soluble in water and may be precipitated using a large counter
anion such as picrate, 12-tungstosilicate, reineckate- or even
perchlorate. In this exercise we will use
12-tungstosilicate.
Dissolve 0.5 g of ferrocene in 10 cm3 concentrated sulfuric
acid; allow the solution to stand for at least half an hour, then
pour it into 150 cm3 distilled water. Stir the solution for a few
minutes and filter off any precipitate. To the filtrate add a
solution of 2.5 g 12-tungstosilicic acid in 20 cm3 water slowly
with stirring. Collect the pale blue precipitate, wash with water
and dry.
b) Acetylation
Ferrocene has an extensive aromatic-type reaction chemistry,
which is reflected in its name and undergoes substitutions more
readily than does benzene. One example is acetylation of both
rings in the presence of a Friedal-Craft catalyst.
Alternatively, the acetylation of ferrocene can be carried out
under milder conditions using acetic anhydride in phosphoric acid
to yield the mono-acetylated product.
References
G.A. Perkins and A.O. Cruz, J. Amer. Chem. Soc., 1927, 49,
517.
Questions
1. Propose a reaction scheme for the acetylations. The 1H nmr
spectra for ferrocene and
a crude mixture obtained from the mono-acetylation using acetic
anhydride are provided. Record the chemical shifts (NB.
TMS=0) and interpret each spectrum.
These experiments are distributed online as a service to our
students and staff. They are designed only for use in our laboratories,
under an appropriate level of supervision by qualified chemistry staff.
Appropriate safety training and proper disposal of chemical waste is assumed.
Other users of the information posted on this site assume all responsibility.
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 1995-2019 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Oct 1995. Links checked and/or last
modified 17th March 2019.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c21jexpt.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page