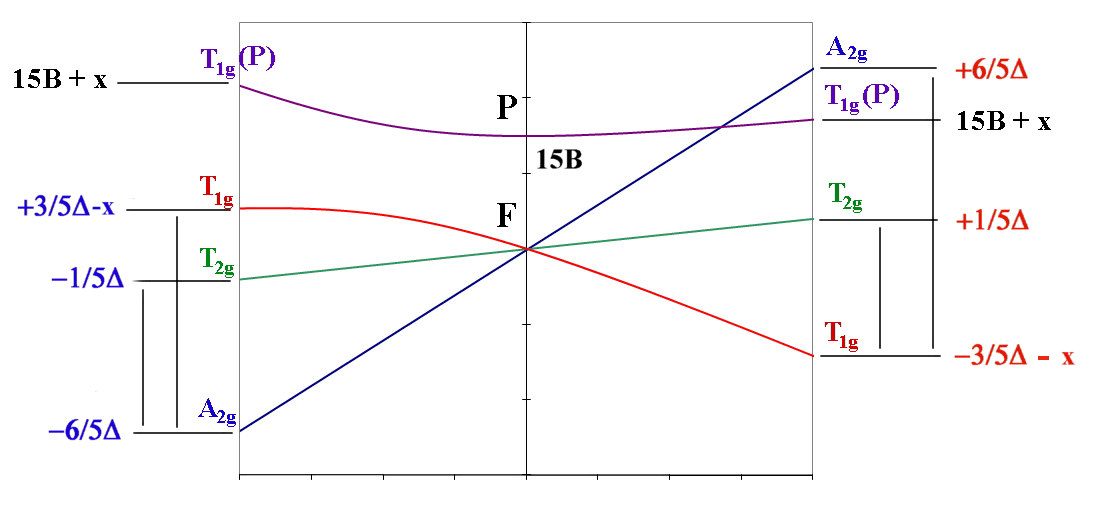
oct d3,d8 tet d2,d7 ←-----------------------------------→ oct d2,d7 tet d3,d8

| ν1 = Δ (T2g←A2g) | ν1= 4/5 Δ + x (T2g←T1g) |
| ν2 = 9/5 Δ - x (T1g←A2g) | ν2 = 9/5 Δ + x (A2g←T1g) OR ν2 = 3/5 Δ + 15B + 2x (T1g(P)←T1g) |
| ν3 = 6/5 Δ + 15B + x (T1g(P)←A2g) | ν3 = 3/5 Δ + 15B + 2x (T1g(P)←T1g) OR ν3 = 9/5 Δ + x (A2g←T1g) |
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2005-2010 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire