Stains, Dyes, Inks and Indicators.
Stains
[Work done in the Department (1950's) on logwood dyes, to go
here.]
Structures of
hematein and
hematoxylin. A brief history of their use is given in
Woods and
Ellis, Laboratory Histopathology: A Complete Reference, 1994
Churchill Livingstone.
These dyes are extracted from haematoxylon campechianum
and are chemically very similar to the dyes
brazilein and
brazilin from the caesalpinia redwoods such as
caesalpinia brasiliensis (which is where the name for
the country Brazil is derived). Another important species is
pernambuco or fernambuco wood (caesalpinia crista) which
is the timber predominantly used for the manufacture of bows for
violins, cellos etc.
Dyes
Annatto
Whilst the use of the dyestuffs from Bixa orellana has
been known for many years, it was only introduced into Europe in
the 16th Century. The crude extract, called annatto, gives rise to a red, light red or
yellow dye and the first isolation of the principal agents was by
Boussingault around 1825.
("Sur les proprietes chimiques du rocou", Ann. Chem., 1825, 28,
440-444.)
It is used extensively in the food industry for dyeing dairy
products (E160b). These dyestuffs are carotenoids and recently a
biosynthetic pathway from lycopene to bixin was identified by
introducing genes into bacteria preengineered to produce
lycopene. It was suggested that bixin-producing
tomatoes would even be feasible in a few years!
Anthocyanines
These are the water soluble compounds responsible for the red to
blue colours in fruits and vegetables such as grapes,
strawberries, apples, red cabbage, etc.
In general they are glycosides of anthocyanidins, where the
sugars are usually glucose, galactose, rhamnose or arabinose. A
Table showing the structures of 18
anthocyanidins is available.
The extinction coefficient for the anthocyanine dye E163 is
reported for a 1% solution in 1 cm cell to be 1120 at 537 nm. A
dilute solution gives the following spectrum, E163.
Beetroot red, betanin
As much as 200 mg of
betanin is found in beetroot.
Cochineal
For some background information on the cochineal insect see the
reports at WebExhibits.org
and U. of
Minnesota.
The
structure of carminic acid is available. Note that both
haematein and cochineal were mentioned by Robert Hooke in 1650
although they both must have been known to the indigenous people
of the Caribbean and Mexico for much longer.
Cochineal and carmine are discussed on the colormaker
site.
Curcumin
Curcumin from turmeric is a yellow
dye
that is extremely heat stable but light sensitive. Best to keep
your curry powders in the dark therefore.
For a range of other spectral files, see the site at Lappeenranta
University of Technology.
The RAMAN spectra of a number of prehistoric dyes and natural
pigments have been recorded and are available as GRAMS .spc files
for download from University
College, London. Permission has been obtained to convert two
of these to JCAMP-DX format, and they are available below,
namely:
saffron and
Prussian Blue.
Indicators
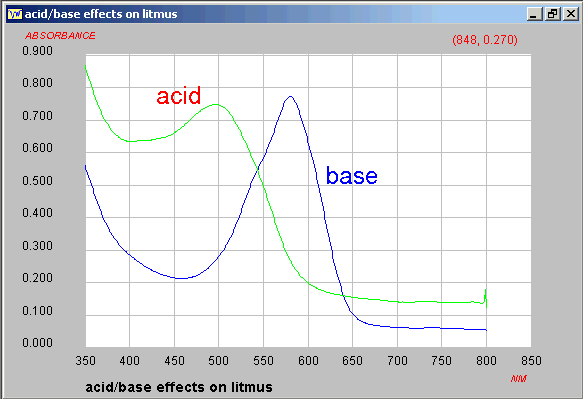
A starting point is "well known litmus", except that the
structure is still not well established since the solution used
in laboratories is a mixture of perhaps more than 6 species.
The visible spectra in
acid or base have been recorded and saved in JCAMP-DX block
format.
The structures
of some common acid base indicators along with the theory
behind their use is presented at Imperial College.
The structure and spectra (in Galactic SPC format) of a range
of dyes is available at The University of
Southern Carolina-Aiken
The history of
dyes from 2600 BC was presented at a gathering of weavers and
spinners in Washington in 1982.
Colouring matter as food additives
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 1997-2014 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created April 1997. Links checked and/or last
modified 8th November 2014.
URL
http://wwwchem.uwimona.edu.jm/lectures/dyesJS.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page