Food Colour and Colour
Additives
The presence of colour in food is a highly motivating force on
our eating habits. Just look at the picture above and decide
which grape or piece of pineapple you want to eat!.
Joint FAO/WHO Expert Committee on Food Additives (JECFA)
The
JECFA has been in existence since 1955 and serves as a
scientific advisory committee to FAO, WHO,
Member Governments and the Codex Alimentarius
Commission. Its principal role is to assess the human health
risks associated with the consumption of additives to food and to
recommend Acceptable Daily Intake (ADI) levels, tolerable limits
for environmental and industrial chemical contaminants in food
and Maximum Residue Levels (MRL) of agricultural chemical inputs
in food such as veterinary drug residues in meat and meat
products.
Colouring matter as food additives
Prior to the discovery of synthetic dyes by Perkins in 1856, only
natural dyestuffs were added to foods. During the early part of
the twentieth century a large number of cheap dyes were
synthesised and many of these then found their way into food
products.
Once countries began to legislate what could be added to food the
number of both natural and synthetic colours used in food dropped
markedly.
The situation today is that countries differ in what they
consider to be safe and the same dyes are not necessarily used
world-wide. In the case of Norway, the use of any synthetic
colour additives has been forbidden since 1976.
The situation becomes even more confusing when natural dyes are
synthesised i.e the nature-identical dyes.
A table showing dyes commonly used as
food additives in a number of different countries are given
with their E, CI and FD&C codes. The E (EEC code) is being
replaced for international use by the Codex Alimentarius
Commission who are developing an International Numbering System
(INS). This will largely use the same numbers (but without the
E). CI is the code used in the Colour Index Volumes.
In the USA, the Food and Drug Administration (FDA) is
responsible for regulating colour additives and they use codes
beginning with FD&C (from the Federal Food, Drug and Cosmetic
Act of 1938).
Tartrazine (CI 19140) for example, is referred to as E102 in
the UK, has an INS number of 102 but in the USA is generally
referred to as FD&C Yellow
No. 5. The JECFA has set its Acceptable Daily Intake (ADI)
value as 0 - 7.5 mg per kilogram of body weight.
Softdrinks
A number of synthetic dyestuffs are commonly found in soft
drinks, for example Kool Aid. For a list of Kool
Aid FAQ which gives the history and describes the flavours
available, check the site in Holland for links.
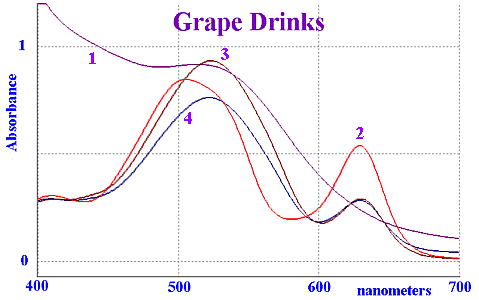 Vis Spectra of "grape" softdrinks
Vis Spectra of "grape" softdrinks
For comparison, some pure grape juice was recorded and is
shown by trace 1. Trace 2 corresponds to Kool-Aid "GRAPE BERRY
SPLASH" TM which contains INS 129 and INS 133. The other two
spectra are local soft drinks that contain INS 123 and INS
133.
These powdered drinks are a convenient source of colouring
material for simple spectrophotometric exercises. The colours
found in packet drinks from a local supermarket include:
| allura red |
INS 129 |
ADI 0 - 7 |
JECFA 25/18 |
| amaranth |
INS 123 |
ADI 0 - 0.5 |
JECFA 25/16 |
| brilliant blue |
INS 133 |
ADI 0 - 12.5 |
JECFA 13/12 |
| fast green FCF |
INS 143 |
ADI 0 - 25 |
JECFA 30/24 |
| sunset yellow |
INS 110 |
ADI 0 - 2.5 |
JECFA 26/24 |
| and tartrazine |
INS 102 |
ADI 0 - 7.5 |
JECFA 8/14. |
Values taken from the Summary of Evaluation performed by the
Joint FAO/WHO Expert Committee on Food Additives 1956-1993 (1st
-41st meetings). The references refer to meeting number / page
number.
For example, the KOOL-AID products "MAN-O-MANGO BERRY" TM and
"SHARKLEBERRY FIN" TM are both listed with FD&C red #40 (ie
allura red). Locally produced GRACE "QUENCH AID" has a drink mix
called "STRAWBERRY RACERS" which contains FD&C red #2 (this
refers to amaranth, banned in the USA since 1976, but used in
Europe as INS 123).
A look at the values above shows the Acceptable Daily Intakes
(ADI) as 0 - 7 and 0 - 0.5 mg/kg body weight respectively.
Problem
- Determine, or look up, the molar absorbances (extinction
coefficients) for these dyestuffs.
Hint. These are sometimes quoted as absorbance for 1%
solutions.
- Calculate the amount of dyestuff in a packet of drink
mix.
- Calculate how many drinks (packets) you would need to exceed
the ADI.
- Email me the answer for your favourite flavour.
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 1997-2015 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created April 1997. Links checked and/or last
modified 10th April 2015.
URL
http://wwwchem.uwimona.edu.jm/lectures/foodcol.html



 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page