Manganese Chemistry
History
For the chapter on Manganese chemistry from the Elsevier text
"Chemistry of the Elements" by Greenwood and Earnshaw see
On-Line Metals Based Surveys
Occurrence
Manganese is the 12th most abundant element and 3rd most abundant transition metal
(cf. Fe, Ti). A number of forms of manganese occur in nature (~ 300 minerals)
giving an overall abundance of 0.106%. 12 of these minerals are economically viable
including: pyrolusite (MnO2), manganite (Mn2O3.H2O),
hausmannite (Mn3O4) rhodochrosite (MnCO3) and Mn-nodules.
The main deposits are found in South Africa and the Ukraine (> 80%) and other
important manganese deposits are in China, Australia, Brazil, Gabon, India, and Mexico.
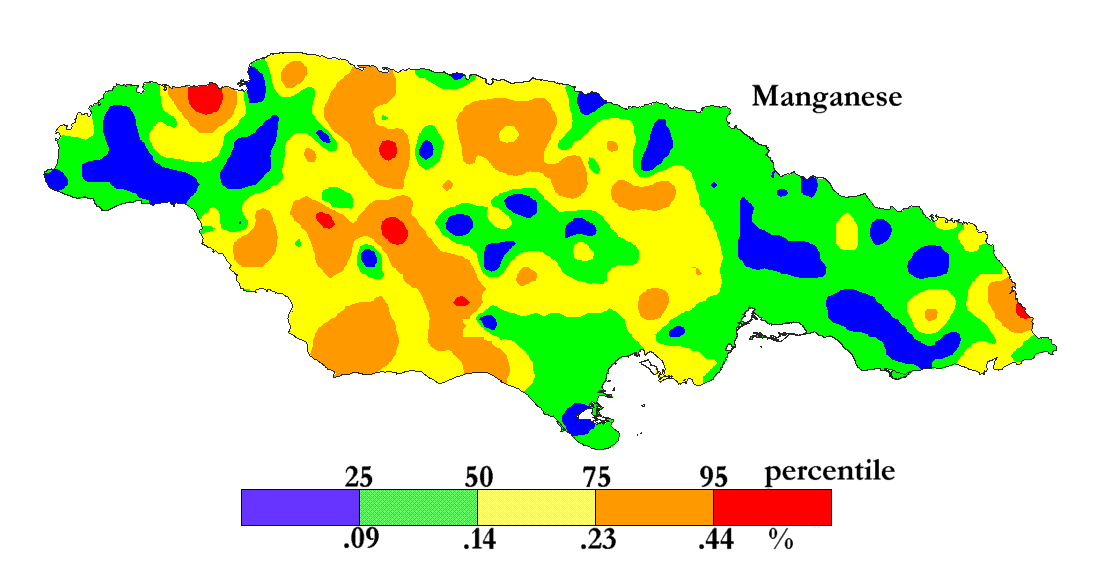
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Manganese are shown below (courtesy of Prof G.C. Lalor).
Extraction
The metal is obtained by reduction with Al, or in a Blast furnace. The metal
resembles iron in being moderately reactive and at high temperatures reacts
vigorously with a range of non-metals.
For example it burns in N2 at 1200 °C to form Mn3N2
and roasting in air gives Mn3O4
Uses
85-90% of the Manganese produced goes in to the fabrication of ferromanganese alloys.
The 1 and 2 Euro coins contain manganese since there it is more abundant and cheaper
than nickel.
Manganese dioxide has been used in the cathodes of dry cell batteries and is used in
newer alkaline batteries as well.
Manganese salts have been used in glass making since the Eygyptian and Roman times
and found in paints from as early as 17,000 years ago.
Its use in glass is either to add colour or to reduce the effect iron impurities
have on the colour of glass, see below.
Manganese in Biology
Manganese is an essential trace element for all forms of life.
It accumulates in mitochondria and is essential for their function.
The manganese transport protein, transmanganin, is thought to contain Mn(III).
Several metalloenzymes are known: arginase, pyruvate carboxylase and
superoxide dismutase.
Humans excrete roughly 10 kg of urea per year, this results from the hydrolysis
of arginine by the
enzyme arginase found on the liver which is the final step of the urea cycle.
This reaction allows for the disposal of nitrogenous waste from the breakdown
of proteins.
In mammalian arginases I and II, binuclear manganese clusters are present
at the active site. In the structure
1rla
the Manganese nearest neighbours were identifed as: Asp124, Asp128, Asp232,
Asp234, His101, His126.
Oxides
Manganese oxides
| Formula |
Colour |
Oxidation State |
MP °C |
| Mn2O7 |
green oil |
Mn7+ |
5.9 |
| MnO2 |
black |
Mn4+ |
535d |
| Mn2O3 |
black |
Mn3+ |
1080d |
| Mn3O4 - Haussmanite |
black |
Mn2/3+ |
1705 |
| MnO |
grey-green |
Mn2+ |
1650 |
Preparations:
Mn3O4 is prepared from the other oxides
by heating in air at 1000 °C
MnO is prepared from the other oxides by
heating with H2 at temperatures below 1200 °C above that Mn metal is produced.
MnO2 has been used for many years to decolourise commercial glass.
When added to molten glass a small amount of red-brown Mn(III) results
that masks the blue-green colour from iron impurities. That is, by adding a
reagent with the complimentary colour of the impurity, the resultant effect
is to balance out and give a clear glass.
MnO2 is used as an oxidant for the conversion of aniline to
hydroquinone.
Mn2O7 is dangerously explosive above 3 °C. It is thought that
some accidents have occurred when instead of adding conc HCl to solid
KMnO4 to produce Cl2 the wrong bottle
is selected and conc H2SO4 was used leading
to the formation of a green oil that explodes.
High Oxidation State Oxide Salts
Fusion of MnO2 with an alkali metal hydroxide and an
oxidizing agent such as KNO3 produces very dark-green
manganate(VI) salts (manganates) which are stable in strongly
alkaline solution but which disproportionate readily in neutral
or acid solution.
3MnO42- + 4H+ →
2MnO4- + MnO2+ 2H2O
The deep-purple manganate(VII) salts (permanganates) may be
prepared in aqueous solution by oxidation of manganese(II) salts
with very strong oxidizing agents such as PbO2 or
NaBiO3. They are manufactured commercially by alkaline
oxidative fusion of MnO2 followed by the electrolytic oxidation
of manganate(VI):
2MnO2+ 4KOH + O2 →
2K2MnO4+ 2H2O
2K2MnO4+ 2H2O →
2KMnO4+ 2KOH + H2
The most important manganate(VII) is KMnO4 and the very intense
purple colour is due to a charge transfer band and not a d-d transition. It is
a well-known oxidizing agent.
Redox properties of KMnO4.
strong base
MnO4- + e- → MnO42- E=0.56V (RAPID)
MnO42- + 2H2O + e- → MnO2 + 4OH- E=0.60V (SLOW)
moderate base
MnO4- + 2H2O + 3e- → MnO2 + 4OH- E=0.59V
dil. H2SO4
MnO4- + 8H2O + 5e- → Mn2+ + 4H2O E=1.51V
The usual conditions for its use are 0.02M KMnO4 and 1.5M H2SO4.
In the industrial production of saccharin and benzoic acid, KMnO4
is the oxidant, medically, it has been used as a disinfectant. It is
gaining in use for water purification, since it has an advantage over chlorine
that it does not affect the taste, and has the bonus that the
MnO2 produced acts as a coagulant for colloidal impurities.
Halides
Manganese(II) halides
| Formula |
Colour |
MP °C |
BP °C |
m (BM) |
Structure |
| MnF2 |
pale-pink |
920 |
- |
- |
rutile |
| MnCl2 |
pink |
652 |
1190 |
5.73 |
CdCl2 |
| MnBr2 |
rose |
695 |
- |
5.82 |
- |
| MnI2 |
pink |
613 |
- |
5.88 |
CdI2 |
Preparations:
Prepared from MnCO3 + HX → MnX2 +
CO2 + H2O
Manganese complexes
Octahedral complexes of Mn(III) are expected to show
Jahn-Teller distortions. It was of interest therefore to compare
the structures of Cr(acac)3 with Mn(acac)3
since the Cr(III) ion is expected to give a regular octahedral
shape. In fact the Mn-O bond distances were all found to be
equivalent.
An unusual Mn complex is obtained by the reaction of
Mn(OAc)2 with KMnO4 in HOAc. This gives
[Mn3O(OAc)6 3H2O]OAc. It is used as an
industrial oxidant for the conversion of toluene to phenol.
return to the C21J course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2003-2007 by Robert John Lancashire, all
rights reserved.
Created and maintained by Prof. Robert J.
Lancashire
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created October 2003. Links checked and/or last
modified 21st November 2007.
URL
http://wwwchem.uwimona.edu.jm/courses/manganese.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page