| At 500 °C 3Fe2O3 +CO → 2Fe3O4 + CO2 Fe2O3 +CO → 2FeO + CO2 |
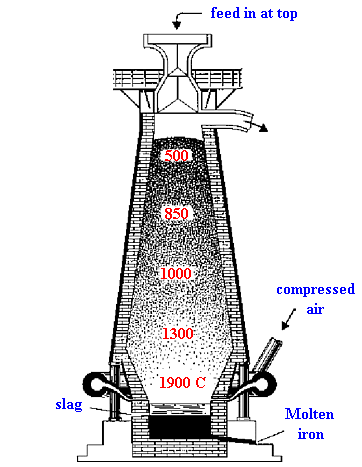 |
| At 850 °C Fe3O4 +CO → 3FeO + CO2 |
|
| At 1000 °C FeO +CO → Fe + CO2 |
|
| At 1300 °C CO2 + C → 2CO |
|
| At 1900 °C C+ O2 → CO2 FeO +C → Fe + CO |
| At 500 °C 3Fe2O3 +CO → 2Fe3O4 + CO2 Fe2O3 +CO → 2FeO + CO2 |
 |
| At 850 °C Fe3O4 +CO → 3FeO + CO2 |
|
| At 1000 °C FeO +CO → Fe + CO2 |
|
| At 1300 °C CO2 + C → 2CO |
|
| At 1900 °C C+ O2 → CO2 FeO +C → Fe + CO |
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2000-2007 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,