
Medieval dyers use long poles to stir cloth in the dye bath to produce red cloth.
From the British Library, Royal Ms 15 E. III f.269 (1482) via Wikipedia

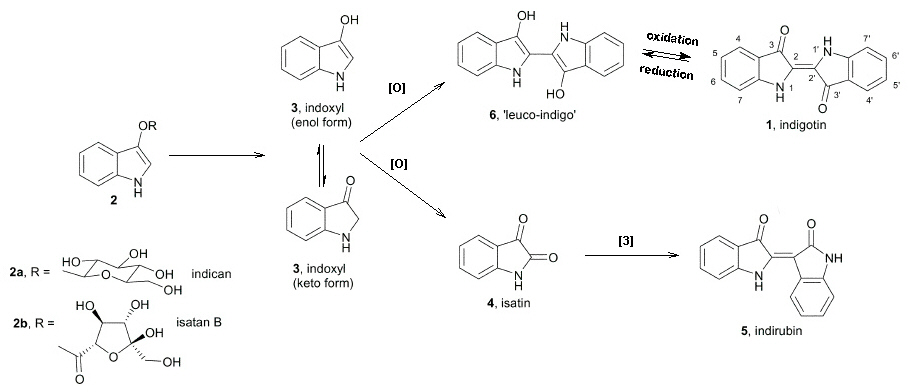
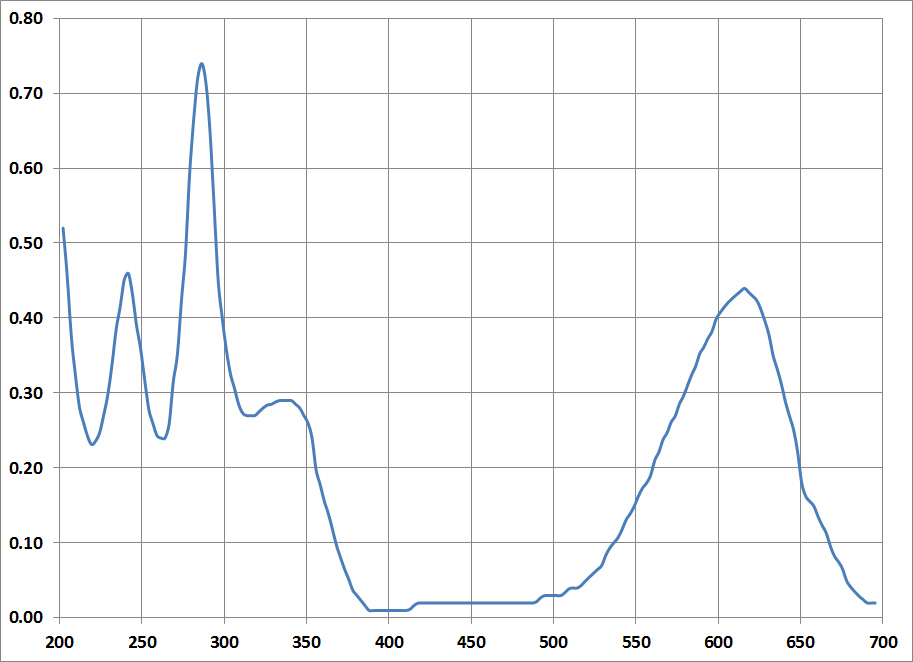
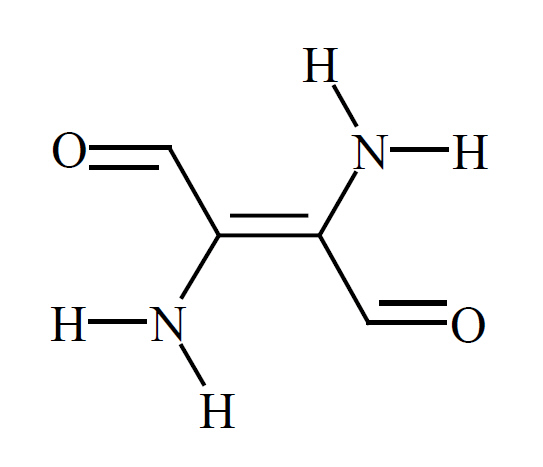
|
|
| possible chromophore and crystal structure of 6,6'-dibromoindigotin | |
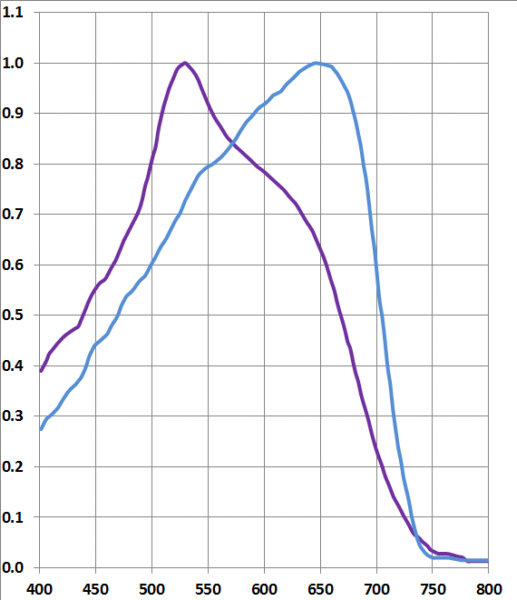
|
|
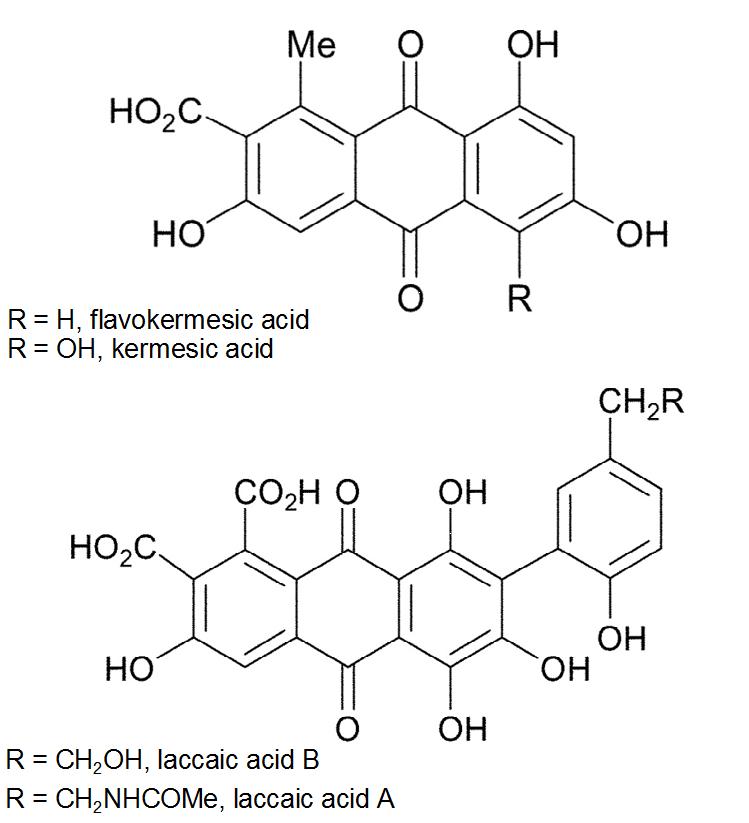
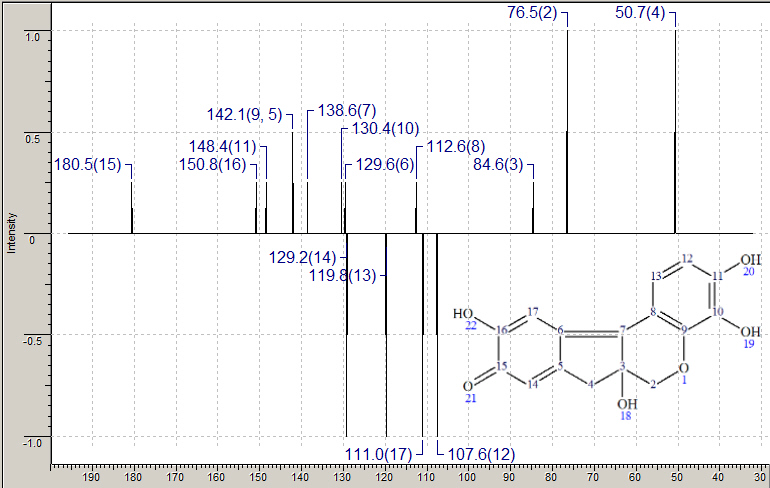
| Red dyes and the structure of carminic acid | |
|
|
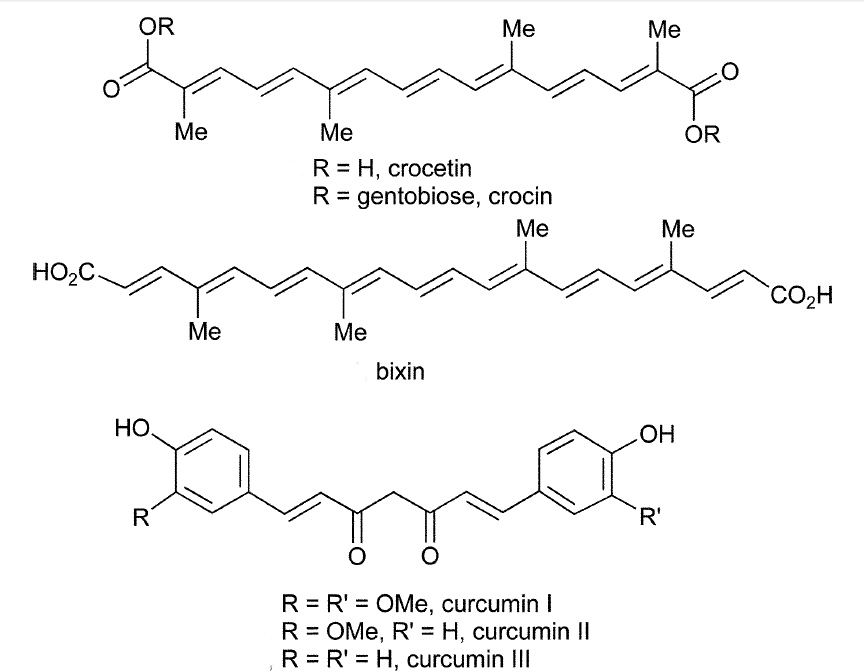
| Yellow dyes and the structure of crocin | |
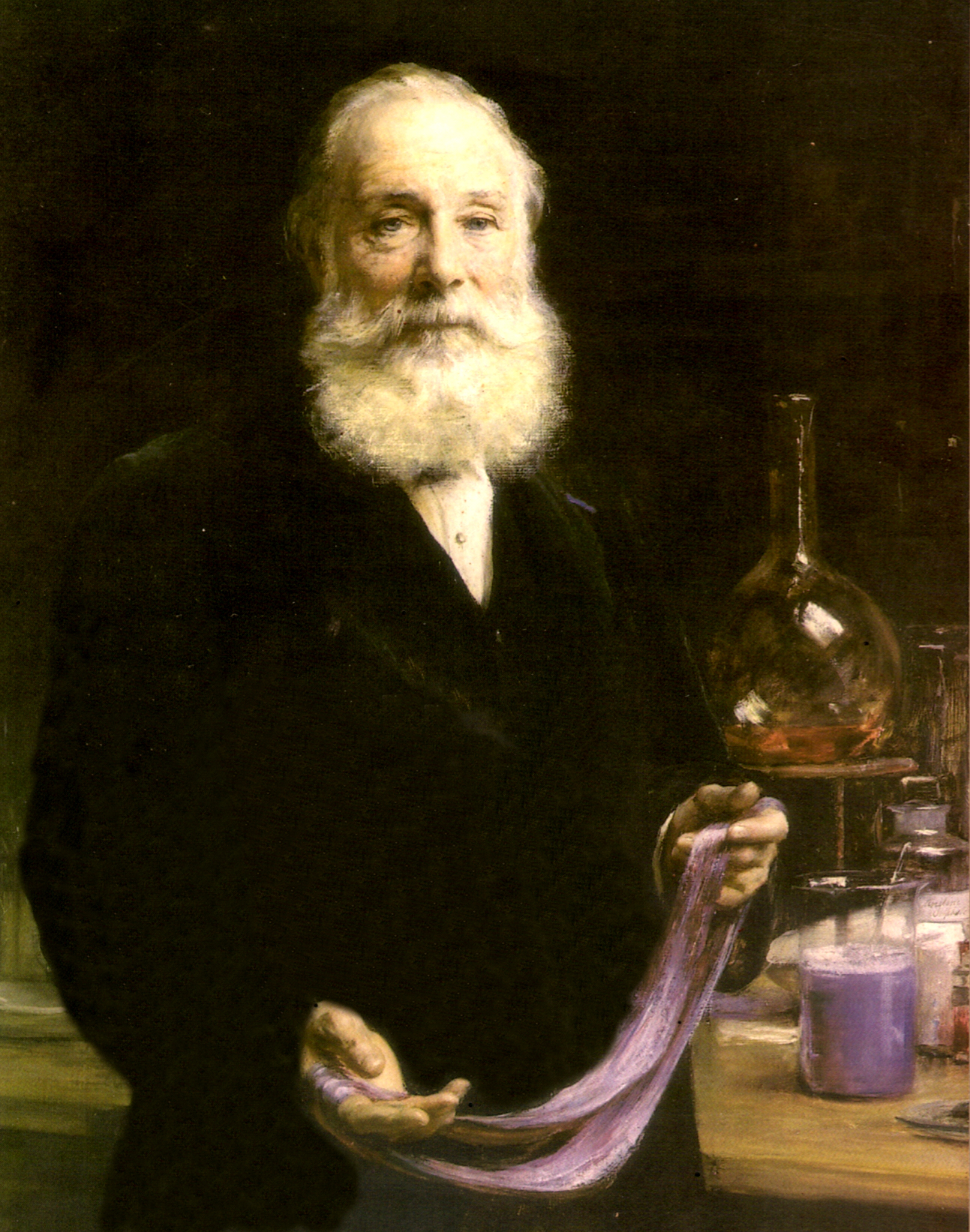
|
|
| William Perkins, who in 1856 (aged 18) discovered the first aniline dye, mauveine. | |
| Year and product | Invented by (date of discovery shown if different from year of first production) |
Synonyms | Procedure |
|---|---|---|---|
| 1858 Tyrian purple |
Perkin (1856) | aniline purple, mauve (1859) | commercial aniline/ potassium dichromate |
| 1859 Fuchsine/aniline red |
Verguin | roseine, rosaniline | commercial aniline/stannic chloride |
| 1860 magenta/aniline red |
Nicholson, Medlock | commercial aniline/arsenic acid | |
| 1861 aniline blue |
Girard and de Laire | aniline red + aniline | |
| 1861 mauve |
Caro (1860) | commercial aniline/copper salts | |
| 1862 aniline black |
Caro | residue of Caro's mauve process | |
| 1862 aniline green |
Usèbe | aldehyde green | aniline red + aldehyde |
| 1862 rosolic acid |
Caro, (Mùller Kolbe and Schmitt; Persoz 1859) | aurin(e), yellow coralline | phenol + oxalic acid + sulfuric acid |
| 1862 cyan brown |
Caro | picric acid + potassium cyanide | |
| 1863 aniline black |
Lightfoot (1859) | direct application of aniline to cotton during printing | |
| 1863 phosphine |
Nicholson | chrysaniline | by-product of magenta manufacture (Nicholson's process) |
| 1863 Hofmann's violets |
Hofmann | trimethylrosaniline and triethylrosaniline | aniline red + alkyl halides |
| 1863/64 induline |
Martius and Caro | azobenzene + aniline | |
| 1863/64 aniline yellow |
Martius and Caro | nitrous acid on aniline | |
| 1863/64 phenylene brown |
Martius and Caro | Bismarck brown (c.1870), Manchester Brown, Vesuvin (BASF) | nitrous acid on m-diaminobenzene |
| 1864 Martius yellow |
Martius and Caro | Manchester yellow, jaune d'or (France), chrysonaphthalic acid, dinitronaphthylalcohol, dinitronaphthalinic acid, binitrohydroxynaphthalene, naphthalene yellow | naphthylamine > diazotise > naphthol + dinitronaphthol |
| Dye | t/year |
|---|---|
| Indigo | 15,000 |
| Disperse blue 79 | 15,000 |
| Sulphur black 1 | 10,000 |
| Reactive dye black 5 | 8,000 |
| Acid black 194 | 7,000 |
|
octaethyl-[22]porphyrin ε= 112000 m2 mol-1 with λmax =460 nm |

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,