

|
|
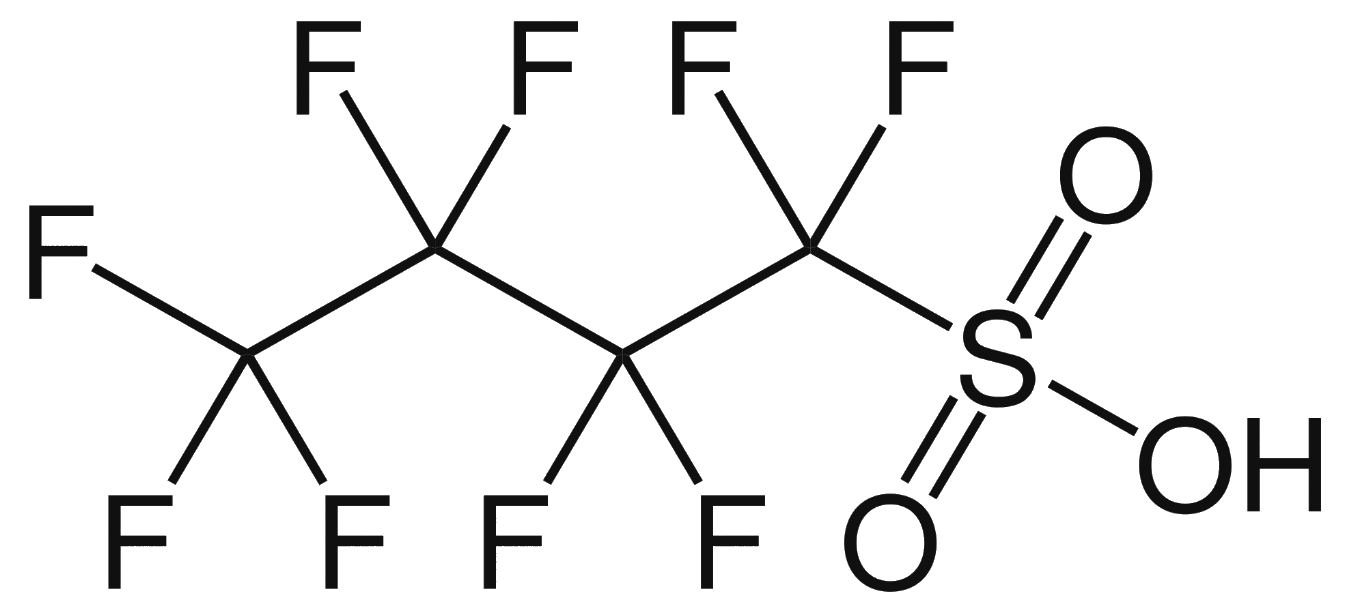
| The structure of perfluorobutanesulfonic acid (PFBS) | |
|
|
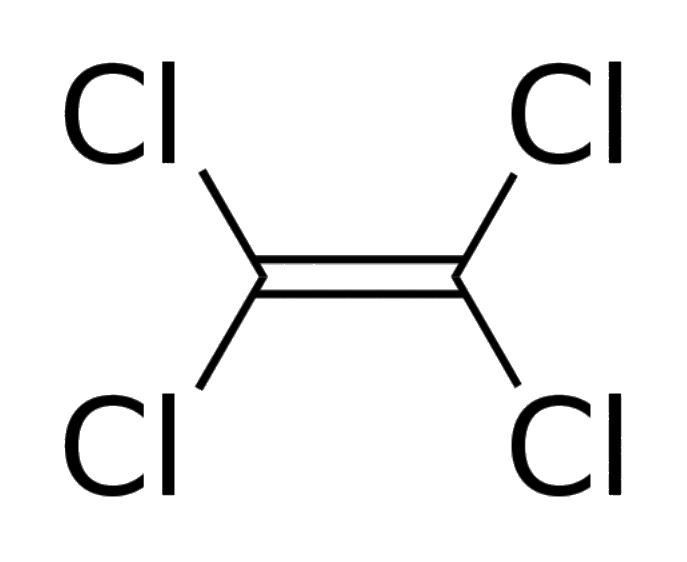
| The structure of tetrachloroethylene (perc) | |
|
|
|
| The structure of Decamethylcyclopentasiloxane (D5), used in "GreenEarth Cleaning" | |

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,