Lecture 5a. Structure of the elements (Groups 1 and 2 metals)
Metallic Structures.
A metal
(from Greek μέταλλον métallon,
"mine, quarry, metal") is a material (an element, compound, or alloy) that is
typically hard, opaque, shiny, and has good electrical and thermal conductivity.
Metals are generally malleable - that is, they can be hammered or pressed
permanently out of shape without breaking or cracking - as well as fusible
(able to be fused or melted) and ductile (able to be drawn out into
a thin wire). About 91 of the 118 elements in the periodic table are metals
(some elements appear in both metallic and non-metallic forms).
Atoms of metals readily lose their outer shell electrons, resulting in a
free flowing cloud of electrons within their otherwise solid arrangement.
This provides the ability of metallic substances to easily transmit heat
and electricity. While this flow of electrons occurs, the solid characteristic
of the metal is produced by electrostatic interactions between each atom and
the electron cloud. This type of bond is called a metallic bond.
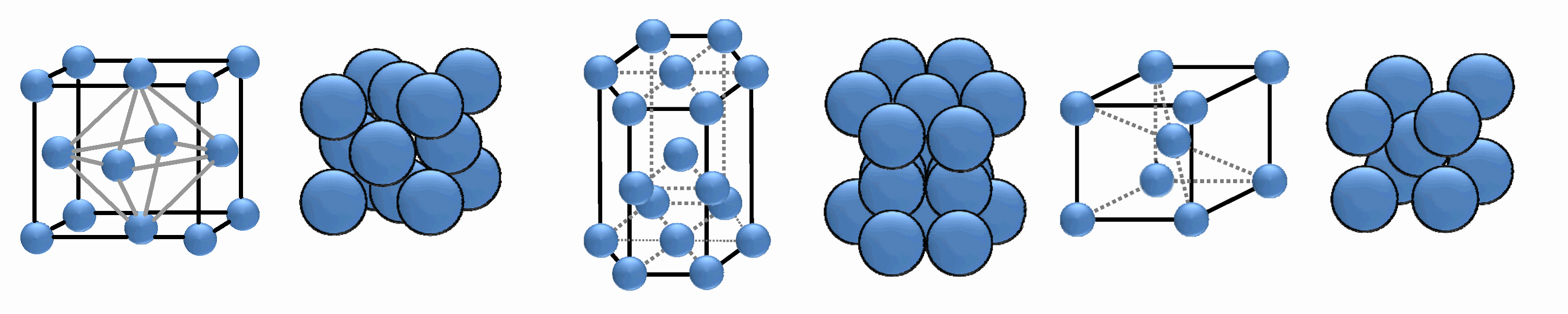
Cubic and hexagonal close packing.
Crystalline solids consist of repeating patterns of its components in
three dimensions (a crystal lattice) and can be represented by drawing the
structure of the smallest identical units that, when stacked together, form
the crystal. This basic repeating unit is called a unit cell.
Many metals adopt close packed structures i.e. cubic close packed (face
centred cubic) and hexagonal close packed structures. A simple model for both
of these is to assume that the metal atoms are spherical and are packed
together in the most efficient way (close packing or closest packing).
For closest packing, every atom has 12 equidistant nearest neighbours, and
therefore a coordination number of 12. If the close packed structures are
considered as being built of layers of spheres then the difference between
hexagonal close packing and cubic close packed is how each layer is positioned
relative to others. It can be envisaged that for a regular buildup of layers:
- hexagonal close packing has alternate layers positioned directly
above/below each other, A,B,A,B,...
- cubic close packed (face centered cubic) has every third layer directly
above/below each other, A,B,C,A,B,C,...
Body centred cubic
This is not a close packed structure. Here each metal atom is at the
centre of a cube with 8 nearest neighbors, however the 6 atoms at the
centres of the adjacent cubes are only approximately 15% further away so
the coordination number can therefore be considered to be 14 when these
are included. Note that if the body centered cubic unit cell is compressed
along one 4 fold axis the structure becomes cubic close packed
(face centred cubic).
Cubic, Hexagonal and Body-centred Packing
cubic close packing (ccp)
packing efficiency =74%
CN=12
|
hexagonal close packing (hcp)
packing efficiency =74%
CN=12
|
body-centred cubic packing (bcc)
packing efficiency =68%
CN=8
|
Trends in melting point
Melting points are chosen as a simple measure of the stability or
strength of the metallic lattice. Some simple trends can be noted.
The transition metals have generally higher melting points than the others.
In the alkali metals (Group 1) and alkaline earth metals (Group 2)
the melting point decreases as atomic number increases, but in transition
metal groups with incomplete d-orbital subshells, the heavier elements
have higher melting points. For a given period, the melting points reach
a maximum at around Group 6 and then fall with increasing atomic number.
Mercury, caesium and gallium have melting points below 30 °C whereas all
the other metals have sufficiently high melting points to be solids at
"room temperature".
The structures of the metals can be summarised by the table below which shows
that most metals crystallise in roughly equal amounts of bcc, hcp and
ccp lattices.
|
Crystal structure of metallic elements in the
periodic table
|
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
H
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
He
|
453.69
Li
bcc |
1560
Be
hcp |
|
MP (K)
At. Symbol
Lattice type |
|
B |
C |
N |
O |
F |
Ne |
370.87
Na
bcc |
923
Mg
hcp |
|
933.47
Al
ccp |
Si |
P |
S |
Cl |
Ar |
336.53
K
bcc |
1115
Ca
ccp |
1814
Sc
hcp |
1941
Ti
hcp |
2183
V
bcc |
2180
Cr
bcc |
1519
Mn
|
1811
Fe
bcc |
1768
Co
hcp |
1728
Ni
ccp |
1357.8
Cu
ccp |
692.68
Zn
hcp |
302.91
Ga
|
Ge |
As |
Se |
Br |
Kr |
312.46
Rb
bcc |
1050
Sr
ccp |
1799
Y
hcp |
2128
Zr
hcp |
2750
Nb
bcc |
2896
Mo
bcc |
2430
Tc
hcp |
2607
Ru
hcp |
2237
Rh
ccp |
1828
Pd
ccp |
1235
Ag
ccp |
594
Cd
|
430
In
|
505
Sn
|
904
Sb
|
Te |
I |
Xe |
301.59
Cs
bcc |
1000
Ba
bcc |
|
2506
Hf
hcp |
3290
Ta
bcc |
3695
W
bcc |
3459
Re
hcp |
3306
Os
hcp |
2719
Ir
ccp |
2041.4
Pt
ccp |
1337.33
Au
ccp |
234.32
Hg
|
577
Tl
hcp |
600.61
Pb
ccp |
544.7
Bi
|
527
Po
|
At |
Rn |
The alkali metals have their outermost electron in an s-orbital and this
electronic configuration results in their characteristic properties.
The alkali metals provide the best example of group trends in properties
in the periodic table, with elements exhibiting well-characterized
homologous behaviour.
The alkali metals have very similar properties: they are all shiny, soft,
highly reactive metals at standard temperature and pressure and readily
lose their outermost electron to form cations with charge +1. They can all
be cut easily with a knife due to their softness, exposing a shiny surface
that tarnishes rapidly in air due to oxidation by atmospheric moisture and
oxygen. Because of their high reactivity, they must be stored under oil
to prevent reaction with air, and are found naturally only in salts
and never as the free element. In the modern IUPAC nomenclature, the
alkali metals comprise the group 1 elements, excluding hydrogen (H),
which is only nominally considered a group 1 element.
Group 2: Alkali earths
extended structures of Li, Mg, Ca
Lithium -bcc
|
Magnesium -hcp
|
Calcium -ccp
|
Note that Housecroft and Sharpe has Ca and Sr both listed as hexagonal and not
cubic (face) close packed lattices. Calcium and Strontium exist in several
allotropic forms and the lowest temperature forms (for Ca < 450 °C) are
ccp. At high temperatures phase transitions occur to give hexagonal.
Return to the
course outline
or move on to Lecture 5: Structure of the elements
Boron, Carbon
and Phosphorus, Sulfur.
References
Much of the information in these course notes has been sourced from Wikipedia under
the Creative Commons License.
'Inorganic Chemistry' - C. Housecroft and A.G. Sharpe, Prentice
Hall, 4th Ed., 2012, ISBN13: 978-0273742753, pps 24-27, 43-50,
172-176, 552-558, 299-301, 207-212
'Basic Inorganic Chemistry' - F.A. Cotton, G. Wilkinson and P.L.
Gaus, John Wiley and Sons, Inc. 3rd Ed., 1994.
'Introduction to Modern Inorganic Chemistry' - K.M. Mackay, R.A.
Mackay and W. Henderson, International Textbook Company, 5th Ed.,
1996.
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created November 2014. Links checked and/or last
modified 17th February 2015.
URL
http://wwwchem.uwimona.edu.jm/courses/CHEM1902/IC10K_MG_struct_elements.html

 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
