
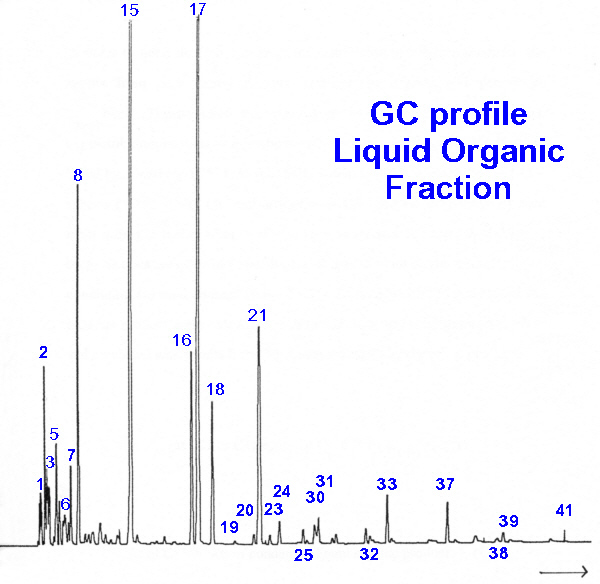
| Peak | Hydrocarbon |
|---|---|
| 1 | 1-butene |
| 2 | 2-methylpropene |
| 3 | 2-methylbutane |
| 5 | 4-methylpentene |
| 7 | methylcyclopentane |
| 8 | benzene |
| 15 | toluene |
| 16 | ethylbenzene |
| 17 | m- and p-xylene |
| 18 | o-xylene |
| 21 | 1-ethyl-4-methylbenzene |
| 24 | 1,2,4-trimethylbenzene |
| 25 | 1-methyl-4-(ethylmethyl)-benzene |
| 30 | 1,2-diethylbenzene |
| 31 | 1-ethyl-2,4-dimethylbenzene |
| 32 | 2,3-dihydro-1-methyl-1-indene |
| 33 | naphthalene |
| 37 | 2-methylnaphthalene |
| 39 | 1,8-dimethylnaphthalene |
| 41 | 2-(1-methylethyl)-naphthalene |
| ZSM-5 sample | CI1/C/H | MZI110/C/H |
| Conversion (%) | >99 | >99 |
| Distribution of conversion products (wt%) | ||
|---|---|---|
| Hydrocarbons | 62.8 | 63.4 |
| Water | 37.6 | 36.9 |
| Distribution of total hydrocarbons (wt %): | ||
| Permanent gases | 58.3 | 46.4 |
| C5+ | 41.7 | 53.6 |
| Selectivity within permanent gases (wt%) | ||
| C1 | 0.3 | 0.1 |
| C2 | 9.8 | 10.3 |
| C3 | 42.6 | 33.3 |
| C4 | 46.9 | 56.3 |
| Selectivity for aromatics in C5+ (wt%) | ||
| Nonaromatics in C5+ | 29.1 | 40.5 |
| Aromatics | 71.9 | 59.5 |
| Benzene | 4.3 | 2.8 |
| Toluene | 21.7 | 11.2 |
| Ethylbenzene | 3.9 | 2.9 |
| m- and p-Xylene | 17.4 | 12.5 |
| o-Xylene | 4.4 | 3.1 |
| EMB** | 5.8 | 10.7 |
| TMB*** | 1.5 | 3.7 |
| 2-Methylnapthalene | 1.0 | 0.4 |
| Others | 11.9 | 12.2 |
a Reactor temperature: 3750C, WHSV:6.48hr-1, N2 flow rate: 20cm3/min, wt of catalyst: 1.0g.
*Zeolite composition:
CI1/C/H-Na0.8H3.4Al4.2Si92O 192.19H2O (Si/Al=21.9)
MZ110/C/H-Na0.1H2.9Al3.0Si93O 192.7H2O (Si/Al=26.3)
Included in the non-aromatic hydrocarbons are:
dimethyl (cyclo) pentanes, methylcyclopentane, ethylcyclopentane and di- and trimethylbutanes, etc.
**EMB: 1-Ethyl-4-methylbenzene
***TMB: 1,2,4-trimethylbenzene
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 1995-2013 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,