Experiment 17 . The preparation of acetanilide from aniline.
(previously listed as Experiment 23)
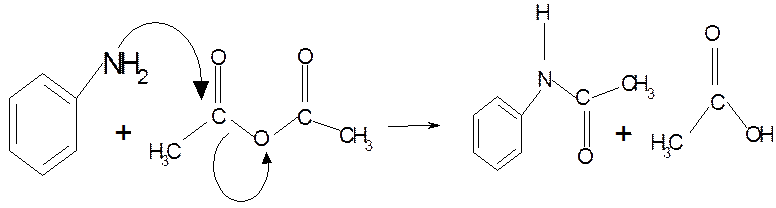
Safety Features:
- 1. Aniline is toxic and can be absorbed through the skin. Use
in a fume hood.
- 2. Concentrated hydrochloric acid can cause severe
burns.
- 3. Acetic anhydride is lachrymatory.
Procedure:
To 60 mL water in a 100 mL Erlenmeyer flask add 2 mL
concentrated hydrochloric acid with mixing.
The next step must be done in the fume hood.
Add 2 mL aniline (density 1.02 g cm-3) and swirl the mixture.
If the solution is coloured, add a small amount of decolourising
charcoal, swirl the flask for about one minute, and filter off
the carbon using a fluted filter paper (see the Appendix).
In a separate container dissolve 3 g sodium acetate
in 10 mL water.
Warm the anilinium chloride solution to 50°C on a
water bath and add 3 mL acetic anhydride (density 1.08 g cm-3).
Swirl to effect dissolution and add the aqueous sodium acetate
quickly. Swirl the flask a couple of times and set it in an
ice-bath for 20 min.
Filter, with suction, the crystals of the amide formed and wash
with a small amount of ice-cold water. Continue to apply suction to the Büchner
funnel for a few minutes.
Dry the material between filter papers and submit your sample for assessment.
Determine the yield and melting point of your product.
Inspect the I.R. spectra of
aniline (starting material) and
acetanilide (product) and record on your worksheet the position of the major bands that
differ between the two (see Appendix 3).
Relate this data to the reaction that has occurred.
Carry out the nitrous acid test on the 1°
aliphatic amine and the on the 1°, 2° and 3° aromatic amines
provided. Record your results in tabular form.
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
Copyright © 1997-2012 by The Department
of Chemistry UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created March 1997. Links checked and/or last
modified 27th March 2012.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c10expt23.html
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page