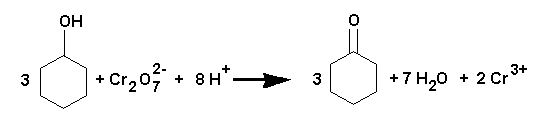
This preparation shows that a ketone can be prepared by the oxidation of a secondary alcohol. In a similar way, an aldehyde can be prepared from a primary alcohol, but since aldehydes are easily oxidised further to carboxylic acids, they must be distilled off from the reaction mixture as formed.
RCH2OH -> RCHO -> RCO2H
(primary alcohol) (aldehyde) (acid)
Pour the mixture into a 250 mL round bottom flask, add water
(60 mL).
Set up a distillation apparatus (but with a stopper
instead of a thermometer and a 100 mL graduated cylinder to
collect the distillate).
Distil the mixture using a Bunsen burner until about 30 mL of distillate
(two layers) has been collected.
Transfer to an Erlenmeyer flask and saturate with salt (about 7 g).
Separate the layer of cyclohexanone in a separatory funnel.
Extract the aqueous layer twice with ethyl acetate (10 mL).
Combine the ethyl acetate extracts with the cyclohexanone layer.
Dry with anhydrous magnesium sulfate (5 min) and filter
into a pre-weighed dry 50 mL RB flask.
Distil off the ethyl acetate, b.p. 77 C, using a distillation
apparatus set up on a steam bath.
This leaves the cyclohexanone (b.p. 153 - 156 C) in the flask.
Determine the weight of the product.
Before discarding your dark green chromium (III) sulfate solution
down the sink, add 2 mL ethanol and swirl.
Inspect the i.r. spectra of cyclohexanol and cyclohexanone. Record on your work sheet the position of the bands and names of the functional groups which change during the oxidation.
For help with interpretation of the IR of cyclohexanol and cyclohexanone check the pages that make use of the JSpecView applet.
Carry out the tests for aldehydes and ketones (Jones and DNP tests) with the samples provided. Record your results in tabular form.
NOTE: Do not stir the mixture with your thermometer!
Explain why ethanol is added to the residue in the distillation flask? Why is this important?
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home PageCopyright © 1997-2009 by The Department of Chemistry UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,