Experiment 12 Preparation of adipic acid from
cyclohexene.
In this laboratory period the cyclohexene prepared in the
previous experiment is oxidised to adipic acid. The procedure is
written for a specific amount of cyclohexene. Before coming to
the laboratory calculate the amounts of reagents required for the
amount of cyclohexene you have available. Show these
calculations in your laboratory worksheet. Alternatively, you may
be supplied with 2 mL of cyclohexene.
Theory:
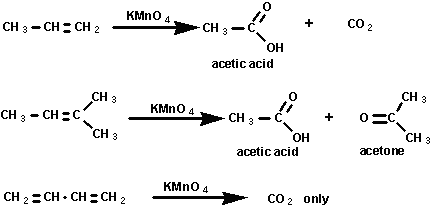
The cleavage of double bonds by oxidation is useful in the
synthesis of acids and ketones and determining structures.
Several methods are available including ozonolysis and hot
concentrated permanganate. The products obtained depend on the
original structure of the olefin. The equations below illustrate
the products from cleavage of alkenes:
The balanced equation for the oxidation of cyclohexene with
permanganate is shown:
Reference:
T.W.G. Solomons, Organic Chemistry, Fourth Edition, p. 322.
Technical Points
Vacuum Drying of Organic Solids
Organic solids may be dried by allowing air to pass through the
filtering apparatus for a few minutes. This, however, only works
well when the solvent used for crystallization has a low boiling
point. A very effective method of rapidly drying materials
isolated from high boiling liquids is to heat them under vacuum.
A simple way of carrying out such an operation on a small
quantity of material involves using a side arm test tube. The
material to be dried is placed in the bottom of such a tube,
which is then stoppered with a cork. The side arm is connected to
the water aspirator. The vacuum is turned on and the test tube is
heated gently in the steam bath. The test tube is shaken
regularly until the solid looks dry and no longer sticks to the
walls of the tube. Remember to always DISCONNECT the hose from
the test tube before turning off the water!
Safety Features:
1. Add concentrated hydrochloric acid to the strongly basic
solutionvery cautiously (or erupting may occur). Wash the
affected area thoroughly with cold water if acid is spilled on
the skin.
2. Handle potassium permanganate carefully. It is a strong
oxidising agent. Avoid contact with skin and eyes.
3. Avoid breathing vapours of cyclohexene and methanol.
Procedure:
To a 250 mL Erlenmeyer flask, add water (50 mL), cyclohexene (2
mL, density = 0.81 g cm-3), and potassium permanganate
(8.4 g). Stopper the flask loosely, wrap with a towel and
swirl vigorously for 5 minutes. The flask should feel warm.
(If no rise in temperature is detected, remove the stopper, warm the
mixture on the steam bath and loosely replace the stopper).
Swirl the flask at frequent intervals for 20
minutes (your yield depends on how well you mix the reactants at
this stage).
The temperature of the mixture should be between 35° and
40°.
If the temperature rises above 45°, briefly cool in
an ice-water bath. Remove the stopper and place the flask on a
steam bath for 15 minutes.
Continue to swirl the flask at frequent intervals.
Make a spot test by withdrawing some of the reaction
mixture on the tip of a stirring rod and touching it to a filter
paper; permanganate, if present will appear as a purple ring
around the dark brown spot of manganese dioxide. If permanganate
is still present, add 1 mL of methanol and heat. Repeat this
procedure until the permanganate colour has disappeared.
Filter the mixture through a large Buchner funnel (vacuum) into a clean
filter flask. Rinse the reaction flask with 10 mL of hot 1%
sodium hydroxide solution and pour through the filter. Repeat
with a second portion of 10 mL of 1% sodium hydroxide solution.
Place the filtrate and washings in a 250 mL beaker (premarked at
10 mL), add a boiling chip and boil over a flame until the volume
of the solution is about 10 mL.
Cool the solution in an ice-water bath and acidify to about pH 1
by cautiously adding concentrated hydrochloric acid
dropwise while stirring the solution. (SAFETY GLASSES!) Add an
additional 3 mL of acid, stir and allow the beaker to stand in
the ice bath for 5 - 10 min to complete the crystallisation.
Collect the acid by vacuum filtration. Recrystallise it from not
more than 5 mL of boiling water (if 2 mL of cyclohexene was
used). Cool to room temperature, then place in an ice-water bath
for 10 minutes.
Filter the product by vacuum (Hirsch funnel) and dry as described
under Technical Points. Measure the melting point and
yield of product. Calculate the percentage yield. Submit your
sample in a labelled vial along with your report.
Infrared Spectroscopy
On your worksheet indicate the important differences in the i.r.
spectrum of the starting material and product. Relate the values
of these absorptions to the structures of the compounds (see Appendix 3).
Animations
of the vibrational modes have been calculated for both
cyclohexene and adipic acid.
NOTE: Stains of potassium permanganate and manganese
dioxide can be removed from equipment, sinks and hands by washing
with a solution of sodium bisulfite (NaHSO3).
Questions:
1. What reaction is effected by the addition of methanol to
unreacted permanganate?
2. Does this reaction affect your product?
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2002-2009 by The Department
of Chemistry UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Nov 2002. Links checked and/or last
modified 19th October 2009.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c10expt12.html

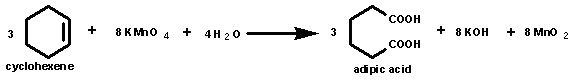



 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page