|
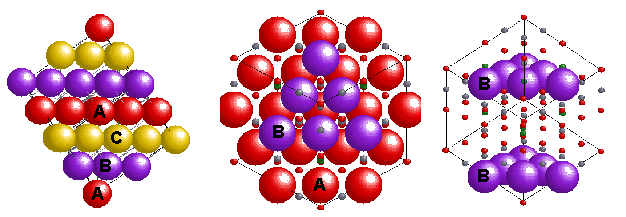
Spinel is the name given to the mineral
MgAl2O4. It has a common structural
arrangement shared by many oxides of the transition metals
with formula AB2O4. In the normal pattern the oxygens (red) form a cubic close packed (face centred) array and the Mg(II) (A(II)-green) and Al(III) (B(III)-silver) sit in tetrahedral (1/8 occupied) and octahedral (1/2 occupied) sites in the lattice, giving a Unit Cell with 8 Mg's, 16 Al's and 32 O's. An inverse spinel is an alternative arrangement where half of the trivalent ions swap with the divalent ions so that the M(II) now occupy octahedral sites ie B(AB)O4.
|
Axes
|
|||||||||||||

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2003 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,