Coordination Numbers and Geometry
Lecture 2. CHEM1902 Coordination Chemistry
The total number of points of attachment to the central element
is termed the coordination number and this can
vary from 2 to as many as 16, but is usually 6.
In simple terms, the coordination number of a complex is influenced
by the relative sizes of the metal ion and the ligands and by electronic factors,
such as charge which is dependent on the electronic configuration of the metal ion.
These competing effects are described by the term ionic potential which
is defined as the charge to radius ratio (q/r).
Based on this, it can be seen that the bigger the charge on the central ion,
the more attraction there will be for negatively charged ligands, however
at the same time, the bigger the charge the smaller the ion becomes which
then limits the number of groups able to coordinate.
Coordination Number 2
This arrangement is not very common for first row transition
metal ion complexes and some of the best known examples are for
Silver(I). In this case we have a low charge and an ion at the
right hand side of the d-block indicating smaller size.
A method that was often employed for the detection of chloride ions involved
the formation of the linear diamminesilver(I) complex.
The first step is:
Ag+ + Cl- → AgCl (white ppt)
and to ensure that the precipitate is really the chloride salt,
two further tests must be done:
AgCl + 2 NH3 → [Ag(NH3)2]+
and
[Ag(NH3)2]+ + HNO3 → AgCl (re-ppts)
The reaction of a bidentate ligand such as 1,2-diaminoethane with
Ag(I) does not lead to chelated ring systems, but instead to linear
two coordinate complexes. One reason for this is that bidentate
ligands can NOT exist in trans arrangements, that is they are
UNABLE to span 180 degrees.
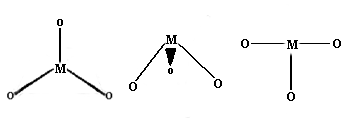
Coordination Number 3
Once again, this is not very common for first row transition
metal ions. Examples with three different geometries have been
identified:
Trigonal planar
Well known for main group species like
CO32- etc., this geometry has the four atoms
in a plane with the bond angles between the ligands at 120
degrees.
Trigonal pyramid
More common with main group ions.
T-shaped
The first example of a rare T-shaped molecule was found in 1977 however
since then several further examples have been reported.
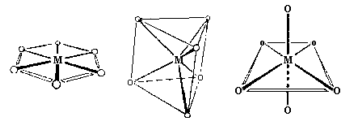
Coordination Number 4
Two different geometries are possible. The tetrahedron is the
more common while the square planar is found in particular with metal ions
having a d8 electronic configuration.
Tetrahedral, (Td)
The chemistry of molecules centred around a tetrahedral C atom is
covered in organic courses. To be politically correct, please
change all occurrences of C to Co. There is a large number of
tetrahedral cobalt(II) complexes known.
Square Planar, (D4h)
These are much less common than tetrahedral and are included here since there
are some extremely important examples with this shape.
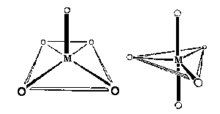
Coordination Number 5
Square pyramid, (C4v)
Oxovanadium salts (Vanadyl, VO2+) often show square
pyramidal geometry, for example, VO(acac)2. Note that
the Vanadium(IV) can be considered coordinatively unsaturated and
addition of pyridine leads to the formation of an octahedral
complex.
Trigonal Bipyramid, (D3h)
The structure of [Cr(en)3][Ni(CN)5] 1.5
H2O was reported in 1968 to be a remarkable example of
a complex exhibiting both types of geometry in the same
crystal.
The reaction of cyanide ion with Ni2+ proceeds via
several steps:
Ni2+ + 2 CN- → Ni(CN)2 yellow
Ni(CN)2 + 2 CN- → [Ni(CN)4]2- orange-red
log(β4) = 30.1
[Ni(CN)4]2- + CN- → [Ni(CN)5]3- deep red
Coordination Number 6
Hexagonal planar
Unknown for first row transition metal ions, although the
arrangement of six groups in a plane is found in some higher
coordination number geometries.
Trigonal prism
Most trigonal prismatic compounds have three bidentate ligands
such as dithiolates or oxalates and few are known for first row
transition metal ions.
Octahedral, (Oh)
The most common geometry found for first row transition metal
ions, including all aqua ions.
In some cases distortions are observed and these can sometimes be
explained in terms of the Jahn-Teller Theorem.
Coordination Number 7
Three geometries are possible:
Not very common for 1st row complexes and the energy difference between
the structures seems small and distortions occur so that prediction
of the closest "idealised" shape is generally difficult.
Capped octahedron, (C3v)
Capped trigonal prism, (C2v)
Pentagonal Bipyramid, (D5h)
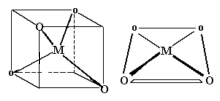
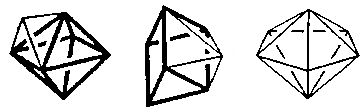
Coordination Number 8
Dodecahedron, (D2d)
Cube, (Oh)
Square antiprism, (D4d)
Hexagonal bipyramid, (D6h)
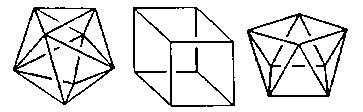
Coordination Number 9
Three-face centred trigonal prism, (D3h)
Coordination Number 10
Bicapped square antiprism, (D4d)
Coordination Number 11
All-faced capped trigonal prism, (D3h)
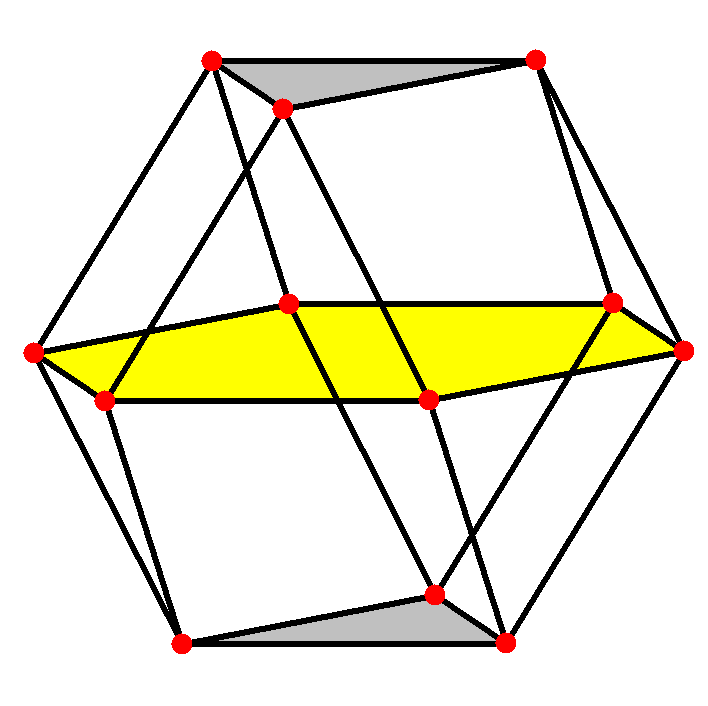
Coordination Number 12
cuboctahedron, (Oh)
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 1996-2015 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created March 1996. Links checked and/or last
modified 9th March 2015.
URL
http://wwwchem.uwimona.edu.jm/courses/IC10Kcnstory.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page