Unit - Chemistry of Garments: Synthetic (man-made) Fibres
Acrylic,
Aramid (
Twaron,
Kevlar,
Technora,
Nomex),
Microfiber,
Modacrylic,
Nylon,
Olefin
Polyester,
Polyethylene (
Dyneema,
Spectra),
Spandex,
Vinylon,
Vinyon,
Zylon
Nylon (Polyamide)
Nylon is a thermoplastic silky material, first commercially used
in a nylon-bristled
toothbrush (1938). Following a US nationwide campaign DuPont sold
around 5 million pairs of "nylons" (stockings) on the 15th May 1940.
The name may have been derived from 'no-run' to emphasise the durability of the
stockings produced from it.
|
|
|
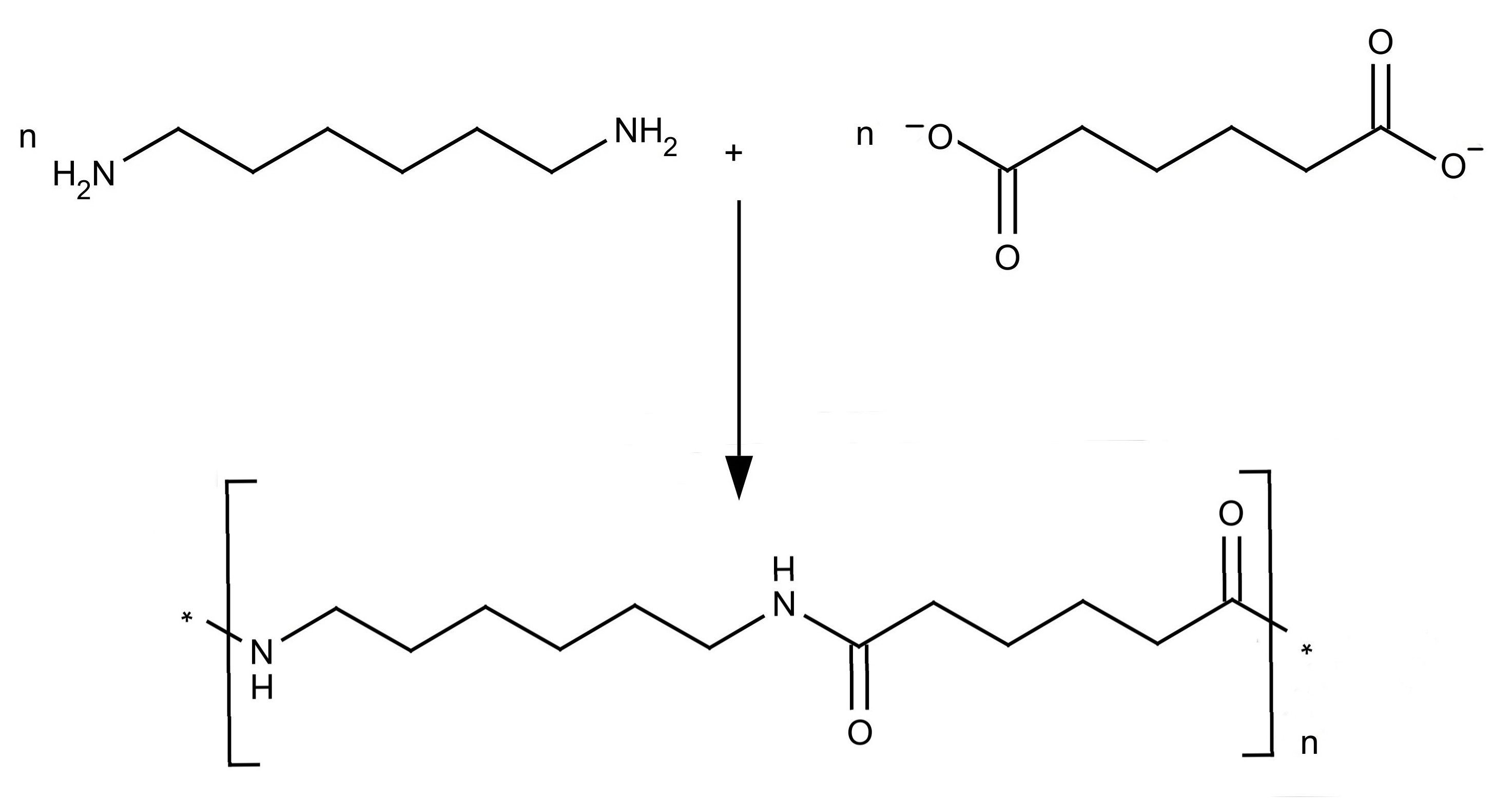
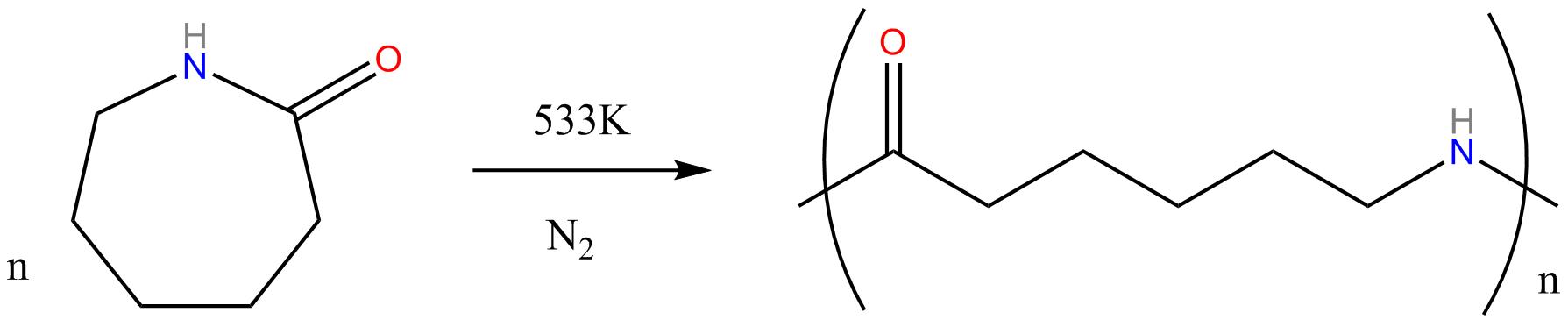
Preparation and structure of Nylon 6 6
|
Nylon was the first synthetic fibre to be made. Nylon-66 and
Nylon-6 are the two important synthetic fibres belonging to the
polyamide group. The numerical suffixes specify the number of
carbons donated by the monomers; the diamine first and the diacid
second. The most common variant is Nylon 6-6 which refers to the
fact that the diamine (hexamethylene diamine, IUPAC name:
hexane-1,6-diamine) and the diacid (adipic acid, IUPAC name:
hexanedioic acid) each donate 6 carbons to the polymer chain. The
Nylon salt so formed on polymerization gives polyhexamethylene
adipamide.
Crystallinity
Note that the molecular graphic images on these pages suggest a
high degree of regular crystallinity which is generally not the
case.
When applied to polymers, the term crystalline has a somewhat
ambiguous usage. In some cases, the term crystalline finds
identical usage to that used in conventional crystallography. For
example, the structure of a crystalline protein or
polynucleotide, such as a sample prepared for x-ray
crystallography, may be defined in terms of a conventional unit
cell composed of one or more polymer molecules with cell
dimensions of hundreds of angstroms or more.
A synthetic polymer may be loosely described as crystalline if it
contains regions of three-dimensional ordering on atomic (rather
than macromolecular) length scales, usually arising from
intramolecular folding and/or stacking of adjacent chains.
Synthetic polymers may consist of both crystalline and amorphous
regions; the degree of crystallinity may be expressed in terms of
a weight fraction or volume fraction of crystalline material. Few
synthetic polymers are entirely crystalline.
The crystallinity of polymers is characterized by their degree of
crystallinity, ranging from zero for a completely non-crystalline
polymer to one for a theoretical completely crystalline polymer.
Polymers with microcrystalline regions are generally tougher (can
be bent more without breaking) and more impact-resistant than
totally amorphous polymers.
Polymers with a degree of crystallinity approaching zero or one
will tend to be transparent, while polymers with intermediate
degrees of crystallinity will tend to be opaque due to light
scattering by crystalline or glassy regions. Thus for many
polymers, reduced crystallinity may also be associated with
increased transparency.
Nylon 6 or
polycaprolactam is a polymer developed at IG Farben to
reproduce the properties of nylon 6,6 without violating the
patent on its production. Unlike most other nylons, nylon 6 is
not a condensation polymer, but instead is formed by ring-opening
polymerization of caprolactam (with 6 C's in the ring, hence the
name).
Although Nylon thread looks delicate, it is in fact as strong as
steel. It has only one-seventh the weight of a steel wire of the
same diameter and hence its tensile strength per unit weight is
superior to that of steel. Because of its great strength, it is
used in making hosiery, textiles, ropes, upholstery, tooth brush
bristles, paint brushes, fishing lines and nets, tennis racquets,
sewing thread, syringes, spectacle frames, zip fasteners and
vehicle tyres blended with rubber. Synthetic grass, which has
been used on tennis courts is made from Nylon. Nylon also
improves the utility of wool fabrics and in mouldable form, it is
used for making bearings and gears, which are self-lubricating.

Preparation of thread of Nylon 6 10 in the Physical Chemistry
Laboratory
Aramids: Kevlar and Nomex
According to the FTC an aramid is defined as:
"A manufactured fibre in which the fibre-forming substance is a long-chain
synthetic polyamide in which at least 85% of the amide (-CO-NH-)
linkages are attached directly between two aromatic rings".
Kevlar
Poly-paraphenylene terephthalamide - better known by the
Trademark, Kevlar - was patented by DuPont in 1962. A research
group at DuPont, began searching for a new lightweight strong
fiber as a substitute to use in the manufacturing of light but
strong tyres having recognised that gasoline resources were
limited. The polymers they had been working with prior to that
time were poly-p-phenylene-terephthalate and polybenzamide, which
formed liquid crystals while in solution, something unique to
those polymers at the time.
In recalling the critical experiment, the discoverer,
Stephanie Kwolek
(1923-2104)
reported that the solution was "cloudy,
opalescent upon being stirred, and of low viscosity" and usually
was thrown away. However, Kwolek persuaded the technician who ran
the "spinneret", to test her solution, and was amazed to find
that the fibre formed did not readily break, unlike nylon. Her
supervisor and her laboratory director understood the
significance of her discovery and within a few years (1971)
modern Kevlar was introduced.
Kevlar's structure consists of relatively rigid molecules which
tend to form mostly planar sheet-like structures similar to silk
protein. It has a high strength/lightweight combination that
makes it the perfect solution for a variety of applications:
- Kevlar K-29 - in industrial applications, such as cables,
asbestos replacement, brake linings, and body/vehicle
armour.
- Kevlar K49 - high modulus used in cable and rope
products.
- Kevlar K100 - coloured version of Kevlar
- Kevlar K119 - higher-elongation, flexible and more fatigue
resistant.
- Kevlar K129 - higher tenacity for ballistic
applications.
- Kevlar AP - has 15% higher tenacity than K-29.
- Kevlar XP - lighter weight resin and KM2 plus fibre
combination.
- Kevlar KM2 - enhanced ballistic resistance for armour
applications
Some of the characteristics that make Kevlar useful in protective
applications:
- Bullet resistant
- Fragment resistant
- Excellent thermal properties
- Lightweight
- Flexible
- Excellent dimensional stability
- Comfortable
- High strength
- Cut resistant
- Chemical resistance
- Puncture resistant
- Slash resistant
- Flame resistant, self-extinguishing
Nomex
Nomex,
which handles similarly to normal textile apparel fibers, is
characterized by its
excellent resistance to heat, as it neither melts nor
ignites in normal levels of oxygen. It is extensively used in the production
of protective apparel, air filtration, thermal and electrical insulation
as well as a substitute for asbestos.
Polyesters
Polyester is made under a range of brand names including: Dacron
(USA), Diolen, Fortel, Kodel, Tergal, Terlenka, Terylene (UK),
Tetoron, Trevira (Germany) and Vycron.

|
|
|
Tall ship replica Surprise, with poly(ethylene terephthalate)
(PET) sails from 125,000 recycled soft-drink bottles.
|
The polyester PET is based on the petroleum products ethylene
glycol and terephthalic acid and is called poly(ethylene
terephthalate). The fibres are linear, long stretched
filamentous molecules. Polyester-cotton blends are popular for
making fabrics, which are used in winter-suits, shirts,
pullovers, socks, carpets, parachute cloth, ropes, wire
insulators, and blankets. Polyesters contribute several
properties to blends, such as wash- and-wear quality,
wrinkle-free texture. Rayon is made from cellulose, cuprammonium
and viscose, it is very cheap and can easily be blended with
other materials as well. Terylene is blended with cotton to
produce what is known as terycot, which is crease resistant and
comfortable as cotton.
The majority of the world's PET production is for synthetic
fibers (in excess of 60%), with bottle production accounting for
around 30% of global demand. In discussing textile applications,
in general PET is referred to as simply "polyester," whereas
"PET" is used most often to refer to packaging applications. The
polyester industry makes up about 18% of world polymer production
and is the third-most-produced polymer; polyethylene (PE) and
polypropylene (PP) are first and second, respectively.
PET
Recycling

Resin identification code used on bottles to allow for recycling of
PET
PET has a
resin identification code of 1. While most
thermoplastics can, in principle, be recycled, PET bottle
recycling is more practical than many other plastic applications.
The primary reason is that plastic carbonated soft drink bottles
and water bottles are almost exclusively PET. It seems as though
only those with resin identification codes 1 and 2 are actually recycled.
The first drink bottles manufactured with PET had round bottoms
and a second plastic (black polyethylene) as a cup on the bottom
to provide a base. This was because the sharp bend needed to make
a flat base weakened the plastic. Current designs mould a bottom
with five, or more convolutions all of which have rounded edges
and this eliminates the need for a second plastic and means that
during the recycling process there is no need to separate the
plastics.
According to the PCI
Group, in 2009 approximately 5.8 million tons of recycled PET
were collected worldwide. From this 4.7 million tons of flake
were obtained. 3.4 million tons were used to produce fibre, 0.5
million tons to produce bottles, 0.5 million tons to produce APET
sheet for thermoforming, 0.2 million tons to produce strapping
tape and 0.1 million tons for miscellaneous applications.
Recycling video
- from water bottles to clothing.
Acrylic Fibres
(polyacrylonitrile, PAN)
Polyacrylonitrile is known by a number of brand names, including:
Acrilan, Creslan, Orlon and Zefran (all of which have 10% to 14%
of other monomers added to improve dyeing), Verel (which is 40%
to 50% vinyl chloride), Dynel (more than 50% vinyl chloride) as
well as Belson, Casmilon, Courtelle, Crylor, Darvan, Teklan and
Vonnel. PAN thus represents a whole family of fibres, which can
be prepared by the polymerization of acrylonitrile. Mixing
ammonia, propylene and oxygen to obtain liquid acrylic nitrile,
which upon polymerization is converted to polyacrylyonitrile, a
synthetic resin powder. As a textile material polyacrylonitrile
is lighter than wool, soft and warm. It can be washed and
drip-dried. It is used as a cheaper substitute of wool for making
warm clothes. Orlon is used in making coats, pullovers, carpets,
and blankets.
Process steps involved in the production of acrylonitrile are:
Catalyst preparation: bismuth and molybdenum
Mixing of propylene, ammonia and oxygen in 1:1:6 ratio
Reaction section: Macrylonitrile, acetonitrile, hydrogen cyanide, unreacted mixture of propylene, ammonia and oxygen are fed to fluidised bed reactor. Various products from reactor are and oxygen. Reaction is highly exothermic.
Removal of ammonia
Absorption of absorbable component from ammonia free gas in water to separate the non-condensable and unconverted propylene, propane, nitrogen, CO and CO2
Stripping of organic components and separation of HCN
Separation of Acrylonitrile and acetonitrile which are close boiling compounds. These are separated by extractive distillation using water as solvent. A dilute solution of acrylonitrile is separated which is recovered and concentrated
Purification of acetonitrile
Final purification of acrylonitrile
Formation of acrylonitrile occurs by the following reactions:
CH2=CH-CH3+ O2 ¾® CH2=CH-CHO + H2O
CH2=CH-CHO + NH3 ¾® CH2=CH-CH=NH + H2O
CH2=CH-CH=NH+ ½ O2 ¾® CH2=CH-CN + H2O
Overall reaction:
CH2=CH-CH3+ NH3+ 3/2 O2¾® CH2=CH-CN + 3 H2O
Side reactions include:
2 CH2=CH-CH3 + 3 NH3 + 3 O2 ® 3 CH3CN + 6 H2O
Russian Journal of Applied Chemistry, Volume 79, Number 8,
1378-1380, DOI:
10.1134/S1070427206080349
Polyacrylonitrile (PAN) is one of the most important and widely
industrially used polymers. It serves to obtain various polymeric
materials with unique properties (thermal stability, strength,
electrical conductivity, hydrophilicity, etc.). This is
determined by specific structural features of the PAN molecule,
whose quantitative and qualitative parameters depend on
preparation techniques and methods of its modification. Various
methods of acrylonitrile polymerization can he used to obtain
polymers with predominance of 3D structures of atactic,
isotactic, and syndiotactic types. Stereoisomers of this kind
possess various properties and differently behave in forming and
under thermal treatment. The "wet" method is for the most part
used in the industry to form PAN fibers, and, therefore, the
spatial orientation of polymer chains of PAN in solution is
important. As a rule, spinning solutions of PAN arc oriented by
mechanical treatment in fibre forming on a technological line.
Tacticity is
a term used to describe the structures of polymers.
Isotactic polymers are composed of
isotactic macromolecules (IUPAC definition). In
isotactic macromolecules all the substituents are located on the
same side of the macromolecular backbone. Isotactic polymers are
usually semicrystalline and often form a helix configuration.
In syndiotactic or syntactic macromolecules the
substituents have alternate positions along the chain. The
macromolecule consists 100% of racemo diads.
In atactic macromolecules the substituents are
placed randomly along the chain. Due to their random nature
atactic polymers are usually amorphous.
In eutactic macromolecules, substituents may
occupy any specific (but potentially complex) sequence of
positions along the chain. Isotactic and syndiotactic polymers
are therefore instances of the more general class of eutactic
polymers.
|
|
|
A syndiotactic fragment of polyacrylonitrile (PAN)
|
Heat treatment of PAN leads initially to the formation of fused
pyridine rings and eventually to carbon
fibre. This has been used to reinforce substances, such as
epoxy resins, where lightness but great strength is required for
example in the turbine blades of jet engines.
Polyolefins
Polyethylene and polypropylene are the lightest fibres and are
generally used in blankets, upholstery, carpets and apparel. They
are difficult to dye given that there are no functional groups to
react with. Polypropylene is also a substitute for sisal in rope
making and in synthetic grass. (Question: What is the material
used at the Mona Hockey Field or the Mona track?).
Ultra-high-molecular-weight polyethylene (UHMWPE, UHMW) is a subset
of the thermoplastic polyethylene. Also known as high-modulus polyethylene,
(HMPE), or high-performance polyethylene (HPPE), it has extremely long chains,
with a molecular mass usually between 2 and 6 million u. The longer chain
serves to transfer load more effectively to the polymer backbone by
strengthening intermolecular interactions. This results in a very tough
material, with the highest impact strength of any thermoplastic presently made.
UHMWPE is synthesized from monomer of ethylene, which are bonded together to
form the base polyethylene product. These ultra-high-molecular-weight
polyethylene molecules are several orders of magnitude longer than those
of familiar high-density polyethylene (HDPE) due to a synthesis process based
on metallocene catalysts, resulting in UHMWPE molecules typically having
100,000 to 250,000 monomer units per molecule each compared to HDPE's 700
to 1,800 monomers.
When formed to fibers, the polymer chains can attain a parallel orientation
greater than 95% and a level of crystallinity from 39% to 75%. In contrast,
Kevlar derives its strength from strong bonding between relatively short molecules.
Dyneema and Spectra are lightweight high-strength oriented-strand gel spun through
a spinneret. They have yield strengths as high as 2.4 GPa (350,000 psi) and
specific gravity as low as 0.97 (for Dyneema SK75). High-strength steels
have comparable yield strengths, and low-carbon steels have yield strengths
much lower (around 0.5 GPa). Since steel has a specific gravity of roughly 7.8,
this gives strength-to-weight ratios for these materials in a range from 8 to 15
times higher than steel. Strength-to-weight ratios for Dyneema are about 40%
higher than for aramids.
UHMWPE fibers are used in armor, in particular, personal armor and on occasion
as vehicle armor, cut-resistant gloves, bow strings, climbing equipment,
fishing line, spear lines for spearguns, high-performance sails,
suspension lines on sport parachutes and paragliders, rigging in yachting,
kites, and kites lines for kites sports. Spectra is also used as a high-end
wakeboard line.
UHMWPE has over 40 years of clinical history as a successful biomaterial for
use in hip, knee, and (since the 1980s), for spine implants. An online repository,
known as the UHMWPE Lexicon, that provides
information and review articles related to medical grade UHMWPE, has been
running since 2000.
Polyurethane
See as well the GB Kauffman
J. Chem. Educ. article on polyurethanes
Methylene
diphenyl diisocyanate (MDI), is an aromatic diisocyanate that
exists as three isomers, 2,2'-MDI, 2,4'-MDI, and 4,4'-MDI with
the 4,4' isomer (InChI:UPMLOUAZCHDJJD-UHFFFAOYSA-N), being the
most widely used. MDI reacts with polyols in the manufacture of
polyurethane. Total world production of MDI and polymeric MDI is
over 2 million tonnes per year (Mt/a) and it accounted for over
60% of the isocyanate global market in the year 2000.
MDI is the least hazardous of the commonly available isocyanates
but that does not imply that it is benign. Its very low vapour
pressure reduces its hazards during handling and this has led to
it being used as a replacement to the other major isocyanate TDI.
2,4-toluene
diisocyanate (TDI) is the second most used aromatic
diisocyanate and it is largely produced for preparing
polyurethanes. It exists in two isomers, 2,4-TDI (InChIKey:
DVKJHBMWWAPEIU-UHFFFAOYSA-N) and 2,6-TDI (InChIKey:
RUELTTOHQODFPA-UHFFFAOYSA-N). 2,4-TDI is produced in the pure
state, but is often marketed as 80/20 and 65/35 mixtures of the
2,4 and 2,6 isomers respectively. It is a highly produced
diisocyanate, accounting for 34.1% of the global isocyanate
market in 2000, second only to methylene
diphenyl diisocyanate (MDI). All major producers of TDI are
members of the International Isocyanate
Institute, whose aim is the promotion of the safe handling of
isocyanates (in particular (MDI and TDI) in the workplace,
community and environment.
A Google preview of the 2003 book
MDI and TDI: Safety, Health and the Environment: A Source Book
and Practical Guide is available from the Wiley website.
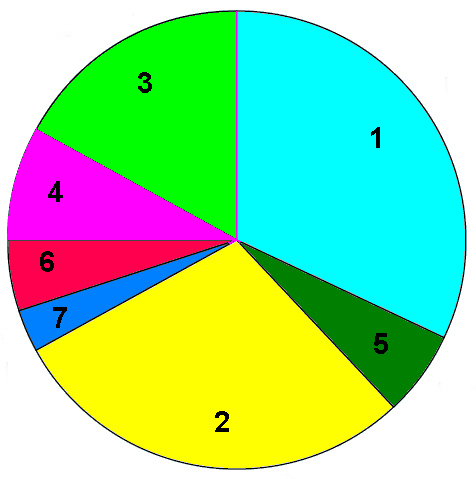
1998 Regional production figures for polyurethane
1) USA and Canada 32%, 2) Western Europe 29%, 3) Asia Pacific
excluding Japan 17% 4) Japan 7%, 5) Latin America 6%, 6) Rest of
the World 5%, 7) Eastern Europe 3%.
Spandex
Spandex is a polyurethane-polyurea copolymer that was co-invented
in 1959 by chemists C. L. Sandquist and Joseph Shivers of DuPont.
Spandex when blended with wool, cotton, nylon, linen or silk
develops properties of stretch and recovery, thus making the
blended fabrics useful for furniture upholstery, light weight
garments and swimsuits.
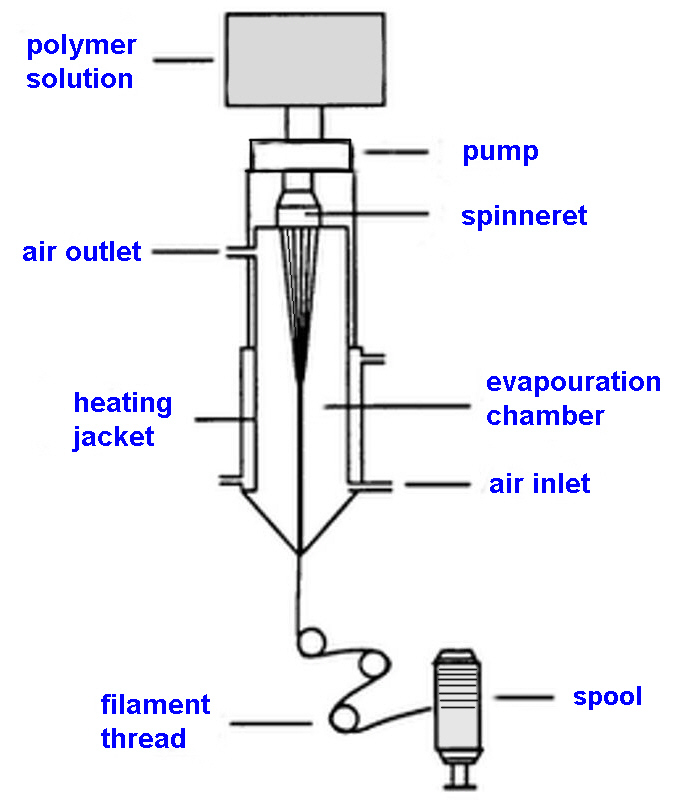
Every superhero has a right to wear Spandex, schematic of the
dry-spinning process.
Spandex fibers are produced in four different ways: melt
extrusion, reaction spinning, solution dry spinning, and solution
wet spinning. All of these methods include the initial step of
reacting monomers to produce a prepolymer. Once the prepolymer is
formed, it is reacted further in various ways and drawn out to
make the fibers. The solution dry spinning method accounts for
over 94.5% of the world's spandex fibers.
Solution dry spinning
Step 1: The first step is to mix a macro glycol with a
diisocyanate monomer in the reaction vessel to produce the
prepolymer. A typical ratio of glycol to diisocyanate is 1:2.
Step 2: The prepolymer is further reacted with an equal amount
of diamine in what is known as a chain extension
reaction. The resulting solution is diluted with a solvent
to produce the spinning solution. The solvent helps make the
solution thinner and more easily handled so it can be pumped into
the fibre production cell.
Step 3: The spinning solution is pumped into a cylindrical
spinning cell where it is cured and converted into fibres. In
this cell, the polymer solution is forced through a metal plate
called a spinneret. This causes the solution to be aligned in
strands of liquid polymer. As the strands pass through the cell,
they are heated in the presence of nitrogen and a solvent gas.
This process causes the liquid polymer to react chemically and
form solid strands.
Step 4: As the fibres exit the cell, an amount of solid
strands are bundled together to produce the desired thickness.
Each fibre of spandex is made up of many smaller individual
fibres that adhere to one another due to the natural stickiness
of their surface.
Step 5: The resulting fibres are then treated with a finishing
agent which can be magnesium stearate or another polymer. This
treatment prevents the fibres' sticking together and aids in
textile manufacture. The fibres are then transferred through a
series of rollers onto a spool.
Step 6: When the spools are filled with fibre, they are put
into the final packaging and shipped to the textile
manufacturers.
Polylactate fibres
It has been reported that a
team of South Korean scientists have produced polymers
useful for everyday plastics through bioengineering, rather than
through the use of fossil fuel-based chemicals. This technique
may allow for the production of environmentally-friendly plastics
that are biodegradable and low in toxicity.
For a review on polylactate
fibres see the article by Jim Lunt and Associates as well as
other
articles on polymers
Non-woven Fabrics
Most non-woven fabrics are made like paper by sticking layers of
fibres together with a liquid adhesive. These fabrics are
commonly used in making hats, bra and shoulder padding,
inner-lining of suits, disposable nappy liners, duvet filling,
furniture upholstery, vacuum cleaner bags and car carpets.
Assignment
The examples provided above are largely based on the same
monomeric reagent polymerising to give homopolymers. Polymers
that are formed containing a mixture of repeat units are known as
copolymers.
- Choose any copolymer
- Describe the applications that it is suitable for
- Describe its structure, for example alternating, random or
block coplymer
- Identify where it is produced and by which company
- Obtain an estimate of the production figures worldwide
- What environmental issues surround its use and disposal?
Acknowledgements.
Much of the information in these course notes has been sourced
from Wikipedia under the Creative Commons License. Students
taking this course will be expected to contribute to Wikipedia as
a part of their course assignments.
Continue to Dyeing Fibres or
return to CHEM2402 course
outline.

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica
Created August 2011. Links checked and/or last
modified 12th September 2014.
URL
http://wwwchem.uwimona.edu.jm/courses/CHEM2402/Textiles/Synthetic_Fibres.html









 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,