Chemistry and Crime
Narcotics and Test Reagent Kits.
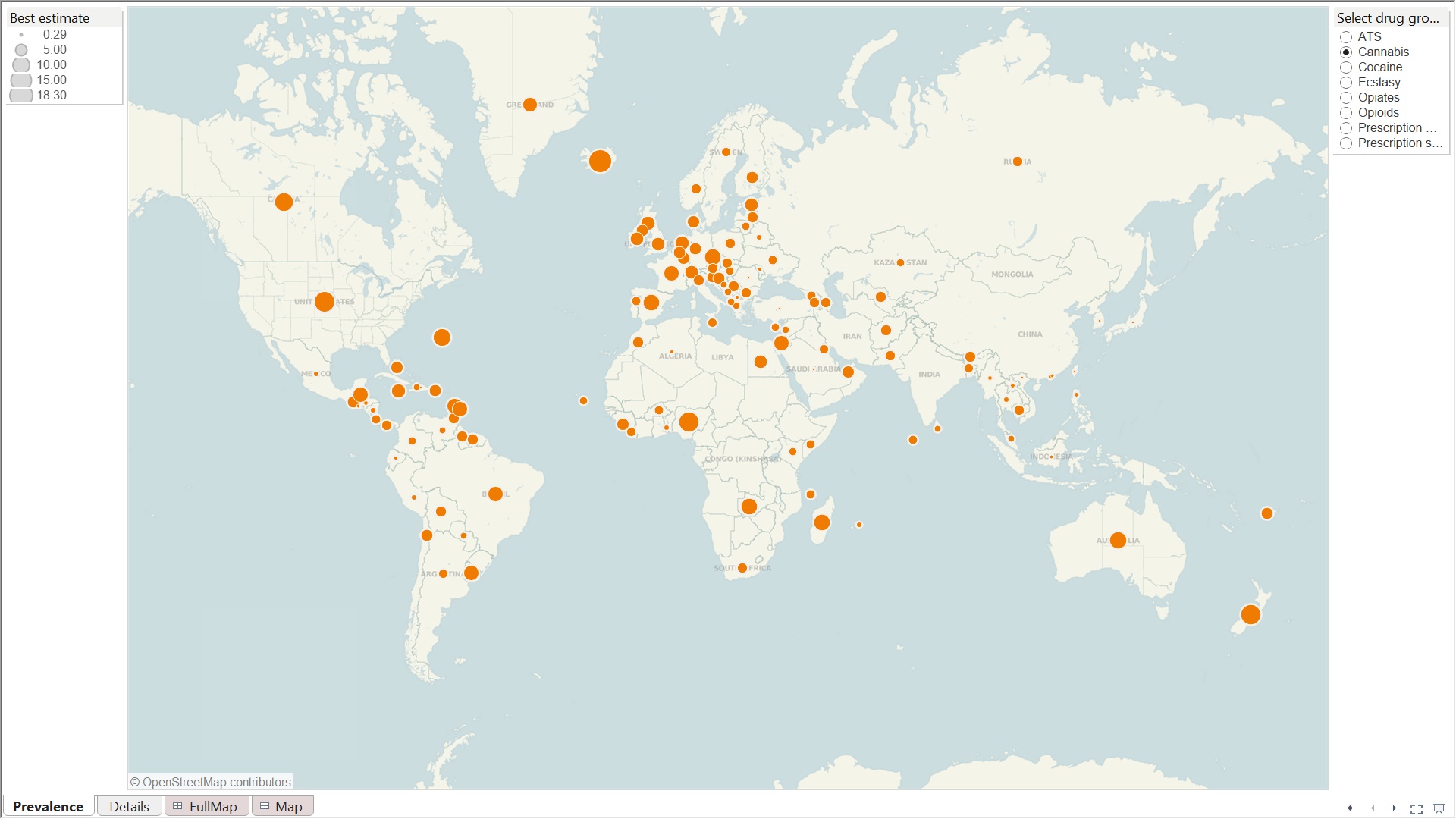
Cannabis useage across the world, 2012
Sources: United Nations Office on Drugs and Crime (UNODC), Annual
Reports Questionnaire Data/DELTA and National Government Reports
The
World Customs Organisation (WCO) Illicit Trade Reports
now cover drugs and tobacco etc. The latest, generally published in June,
(2013 report) is available. Initially these analysed seizures on
a regional basis and by category of drug intercepted,
and focussed in particular on routings, means of transport and
concealment methods used. Emphasis was placed on the illegal
trade in opiates, cocaine, cannabis, and psychotropic substances.
The 2009 figures showed that there were 14,127 seizures of drugs, yielding
a total of 533 tonnes of narcotics, including 43 tonnes of cocaine, 408
tonnes of cannabis and 21 tonnes of opiates. The number of
seizures in 2009 remained relatively stable compared with 2008,
although there was a decrease in the quantities of drugs
intercepted (533 cf. 600 tonnes).
| Regional Intelligence Liaison Offices (RILO) |
2007 |
2008 |
2009 |
2011 |
2012 |
| Asia and the Pacific |
1,353 |
1,476 |
1,879 |
1,320 |
2,16 |
| Caribbean |
51 |
41 |
136 |
25 |
44 |
| Central Africa |
9 |
2 |
6 |
19 |
10 |
| CIS Region (Commonwealth of Independent States) |
429 |
447 |
461 |
1,137 |
1,256 |
| Eastern and Central Europe |
223 |
250 |
291 |
339 |
471 |
| Eastern and Southern Africa |
75 |
115 |
62 |
43 |
20 |
| Middle East |
164 |
207 |
273 |
1,151 |
989 |
| North Africa |
405 |
389 |
359 |
125 |
86 |
| North America |
537 |
472 |
na |
32,752 |
29,690 |
| South America |
614 |
753 |
893 |
746 |
541 |
| West Africa |
64 |
102 |
78 |
72 |
129 |
| Western Europe |
9,443 |
10,001 |
9,689 |
9,427 |
7,963 |
| Grand Total |
13,367 |
14,255 |
14,127 |
47,156 |
43,385 |
Globally, the United Nations Office on Drugs and Crime (UNODC)
estimated that, in 2009, between 149 and 272 million people, or
3.3% to 6.1% of the population aged 15-64, used illicit
substances at least once in the previous year. About half that
number were estimated to have been current drug users, that is,
having used illicit drugs at least once during the past month
prior to the date of assessment. While the total number of
illicit drug users has increased since the late 1990s, the
prevalence rates have remained largely stable, as has the number
of problem drug users*, which is estimated at between 15 and 39
million.
*While there is no established definition of
problem drug users, they are usually defined as those who
regularly use illicit substances and can be considered dependent,
and those who inject drugs.
Cannabis is by far the most widely used illicit drug type,
consumed by between 125 and 203 million people worldwide in 2009.
This corresponds to an annual prevalence rate of 2.8%-4.5%. In
terms of annual prevalence, cannabis is followed by ATS
(amphetamine-type stimulants; mainly methamphetamine, amphetamine
and ecstasy), opioids (including opium, heroin and prescription
opioids) and cocaine. Lack of information regarding use of
illicit drugs - particularly ATS - in populous countries such as
China and India, as well as in emerging regions of consumption
such as Africa, generate uncertainty when estimating the global
number of users. This was reflected in the wide ranges of the
estimates.
While there are stable or downward trends for heroin and cocaine
use in major regions of consumption, this is being offset by
increases in the use of synthetic and prescription drugs.
Non-medical use of prescription drugs is reportedly a growing
health problem in a number of developed and developing countries.
Narcotic Reagent
Kits
Identification of narcotics or other controlled substances can be
a difficult task. There are many potential methods and formulae,
that all require knowledge of chemistry and laboratory
techniques. For quick presumptive testing, colour spot tests for
identification purposes are typically used, and these are
generally completed outside of a laboratory.
The kits are designed so that there is no need for measurement of
chemicals or special equipment. In each plastic tube are
crushable glass ampoules with the complete chemistry for each
test. The specified amount of suspect material is added and the
test is then performed according to the instructions. A typical
set of reagent test kits are shown below:
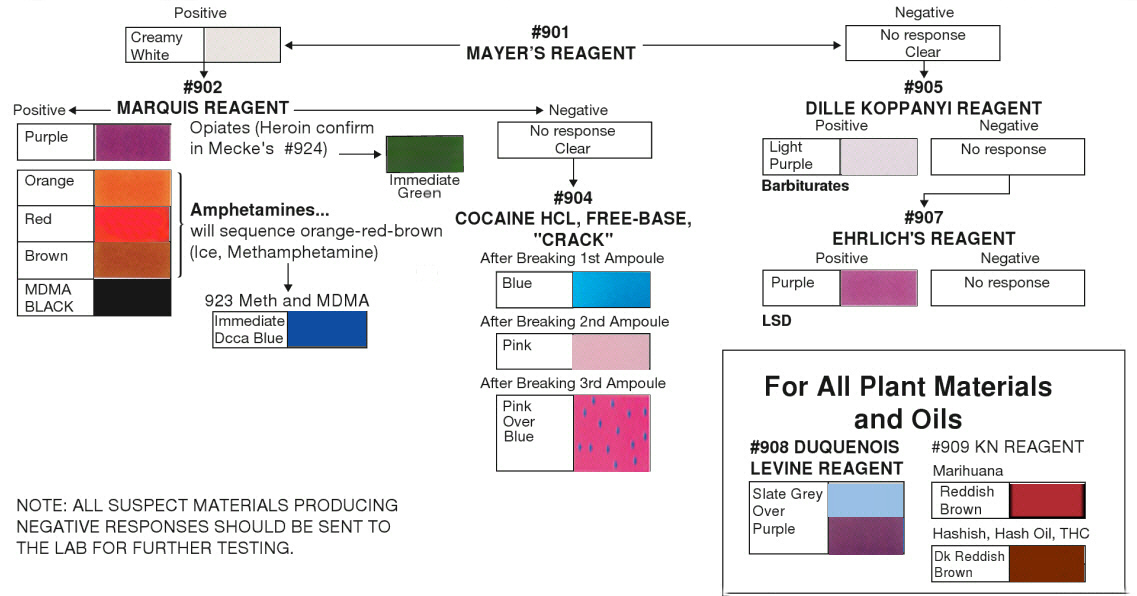
Colour coded results for narcotic tests
| --TEST-- |
REAGENT |
SUBSTANCE PRESUMPTIVELY IDENTIFIED |
| No. 1 |
Mayer's Reagent |
Alkaloids,
Amphetamines |
| No. 2 |
Marquis' Reagent |
Amphetamines, Heroin, MDMA (Ecstasy) |
| No. 3 |
Nitric Acid Reagent |
Heroin, Morphine |
| No. 4/13 |
Cobalt-Thiocyanate Reagent |
Cocaine HCl, Freebase, Crack |
| No. 5 |
Dille-Koppanyi Reagent |
Barbiturates |
| No. 6 |
Mandelin Reagent |
Amphetamines |
| No. 7 |
Ehrlich's Reagent |
Hallucinogens |
| No. 8 |
Duquenois-Levine Reagent |
Marijuana, THC |
| No. 9 |
KN (Fast Blue B Salt) Reagent |
Marijuana, THC |
| No. 14 |
Valium Reagent |
Valium, Flunitrazepam (Rohypnol), Ketamine |
| No. 15 |
Methamphetamine,
Simon's and Robadope's Reagent |
Methamphetamine, MDMA (Ecstasy) |
| No. 16 |
Mecke's Modified Reagent |
Heroin (White, Black, Brown) |
| No. 18 |
Talwin Reagent |
Talwin |
| No. 19 |
Ephedrine Reagent |
Ephedrine |
| No. 22 |
Special Opiates |
Heroin, Oxycodone |
| No. 29 |
PCP Reagent |
PCP, Methaqualone |
Mayer's
Reagent
A precipitating reagent for alkaloids prepared from a solution of
mercuric chloride and potassium iodide in deionized water.
Isoquinoline alkaloid sub-structure and Mayer's Reagent
Alkaloid + K2[HgI4] ↔
[Alkaloid-H+][HgI3]- ↓ (or
[HgI4]) salt
The formation of a creamy-white precipitate is indicative of the
presence of one of the narcotic alkaloids or the amphetamines.
This is often the first screening test performed and depending on
the result may lead to further testing with Marquis' Reagent or
the Dille-Koppanyi Reagent.
Marquis'
Reagent
The Marquis reagent is a spot test for alkaloids that was first
reported in 1896. The original testing agent was a mixture of 2
drops of 40% formaldehyde and 3 milliliters of concentrated
sulfuric acid. It was originally used for detecting small amounts
of certain alkaloids, and for distinguishing between them. The
signature of the alkaloid is both the initial colour produced, as
well as the sequence of colour changes occurring with time. In the
early days the Marquis reagent was used primarily to distinguish
the opium alkaloids. Each alkaloid had a pattern of colour change.

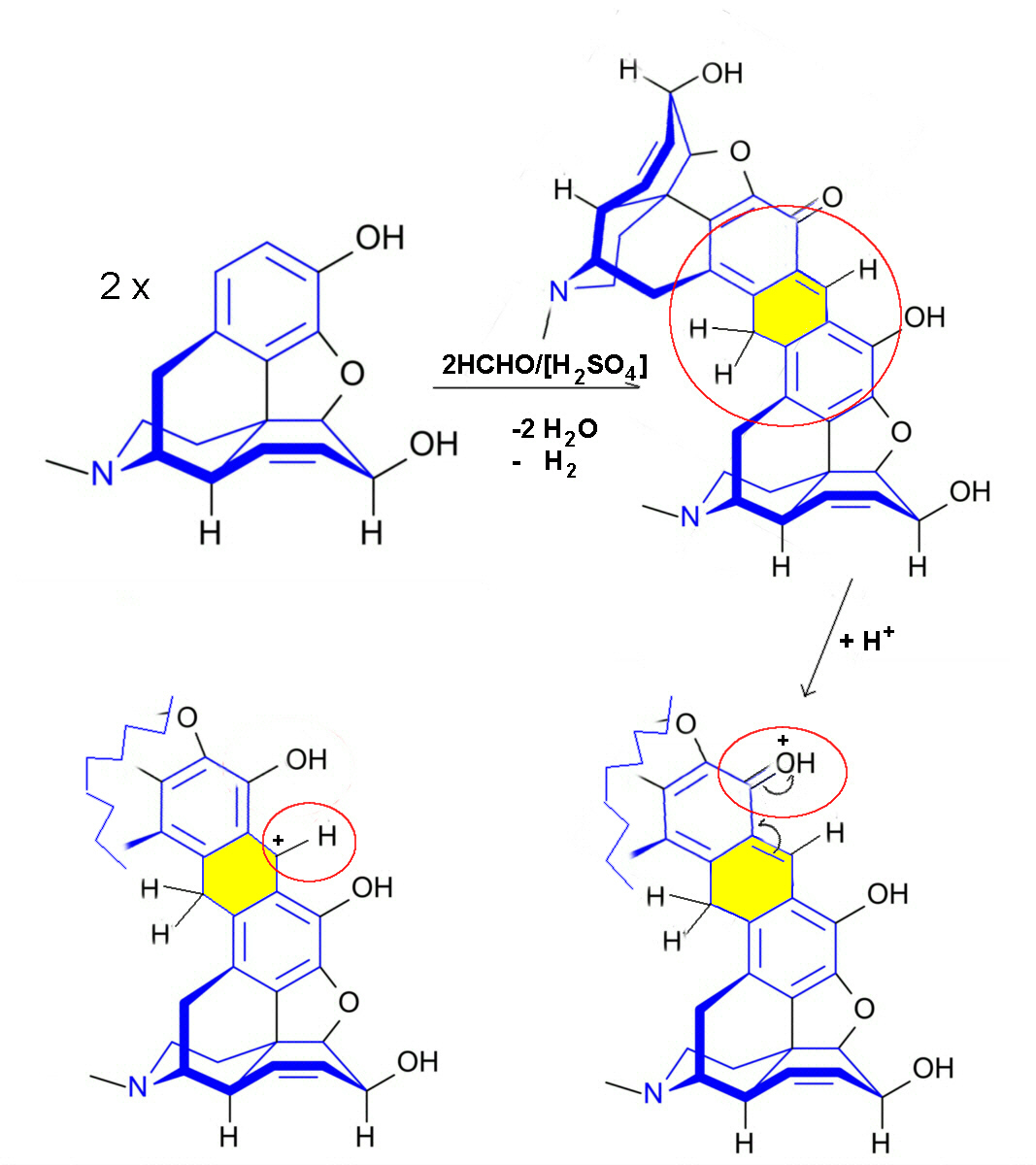
Morphine reaction with Marquis' reagent
The colour reaction of morphine with Marquis' Reagent results in
a purple to violet colouring. It is proposed that two molecules
of morphine and two molecules of formaldehyde condense in the
presence of conc. sulfuric acid to the dimeric product which is
protonated to the oxoniumcarbenlum salt.
Cobalt
Thiocyanate Reagent, Scott's test
Cobalt thiocyanate in aqueous solution and stannous chloride in
aqueous solution.
Various field tests are used to identify cocaine/crack in
apprehended street samples. Most of them are based on
complexation of the alkaloid with cobalt(II) thiocyanate (Co(SCN)2(H2O)4)
solution which results in the formation of
a blue colour. The cobalt thiocyanate test was developed in 1973
and later improved to make it applicable to crack.
A recent article highlighted the use of
TLC for detection of cocaine and suggested the blue colour is a
6 coordinate Co(II) species with one isothiocyanate ligand
a bidentate cocaine molecule and 3 waters coordinated?

Scott's Reagent
See as well a
study on the variation of acids used for acidifying the
solution and the
effect of temperature on the test.
A suggested reaction is the change from octahedral pink Co(II) to
tetrahedral blue Co(II) similar to that seen for the chloride
salts.
[Co(SCN)(H2O)5]+(aq) + 3
SCN-(aq) + 2 R3NH+ ↔
(R3NH)2[Co(SCN)4] + 5
H2O(l)
where [R3NH]+ represents the protonated
cocaine ion.
At 4°C the sensitivity of the test was found to double
compared to room temperature (22°C), while temperatures in
excess of 40°C decreased the sensitivity of the test more
than twofold versus room temperature. These findings clearly show
the impact that storage, use, and interpretation of commercially
available cocaine test kits in typical field settings may
experience in very hot ambient temperatures.
Dille-Koppanyi Reagent
The original 2 part test was developed in the 1930s by American pharmacologists
Theodore Koppanyi and James M. Dille as a simple spot-test to presumptively
identify barbiturates.
Part A is 0.1 g of cobalt(II) acetate dihydrate dissolved in 100 ml of
methanol mixed with 0.2 ml of glacial acetic acid. Part B is made up of
5% isopropylamine (v/v) in methanol. Two drops of A are addeded to
the substance followed by one drop of B and any colour changes observed.
The test turns phenobarbital, pentobarbital, amobarbital and secobarbital
light purple.
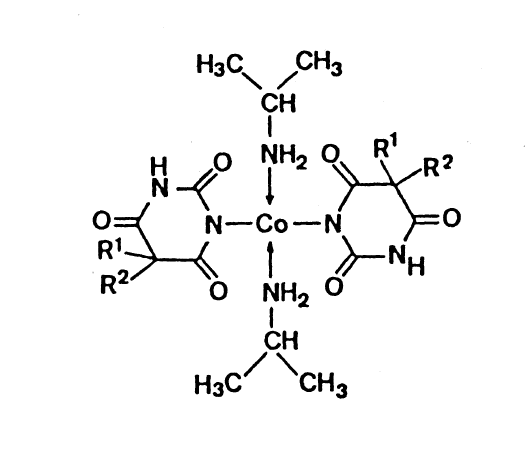

Dille-Koppanyi Reagent test
N-non-substituted barbiturates can be detected with
Dille-Koppanyi's Reagent. Isopropylamine is responsible for the
deprotonation of the barbiturate molecule. The purple colour is
caused by complex formation between two barbiturate molecules,
two isopropylamine molecules around a tetrahedral cobalt(II). The
isopropylamines are thought to act as stabilizers of the complex.
Mandelin Reagent
The Mandelin reagent is used as a simple spot-test to presumptively
identify alkaloids as well as other compounds. It is prepared by
the addition of 100 mL of concentrated (95-98%) sulfuric acid to
1 g of ammonium vanadate.
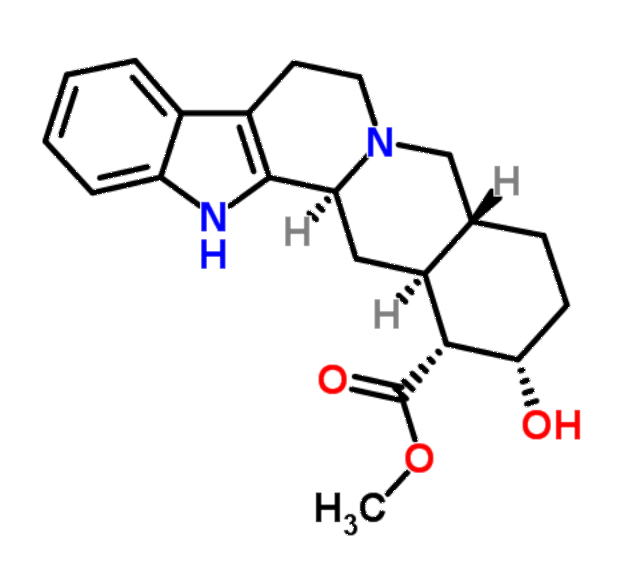
The alkaloid Yohimbine and Mandelin Reagent
According to the
Journal of the American Pharmaceutical Association Volume 19,
Issue 12, pages 1292-1299, December 1930
"An ammonium vanadate-sulfuric acid solution, as a test for
strychnine was first proposed by Mandelin in 1883. A few
alkaloids besides strychnine will give colour reactions with this
reagent. According to Witthaus the following alkaloids give
strychnine-like reactions with this reagent:
1. Curarin gives about the same play of colours, but the
appearance is very much delayed. Curarin, however, is not
extracted with organic solvents in alkaline solutions.
2. Gelsemin produces a purple or red-violet colour.
In 1915, it was shown that yohimbine, an alkaloid obtained from
the bark of Corynanthe Johimbe, gives the same purple
with Mandelin's reagent as strychnine. However upon dilution with
water the purple colour developed by strychnine changes to a
beautiful rose-pink colouration, whereas with yohimbine no such
colour develops upon dilution.
In 1889, a number of alkaloids were tested with Mandelin's
reagent. Distinctive colours with twenty-three of the common
alkaloids were obtained. Apart from strychnine, violet or
violet-blue colours were obtained with apomorphine and
papaverin."
Ehrlich's
Reagent
A solution of p-dimethylamino benzaldehyde and concentrated
hydrochloric acid.
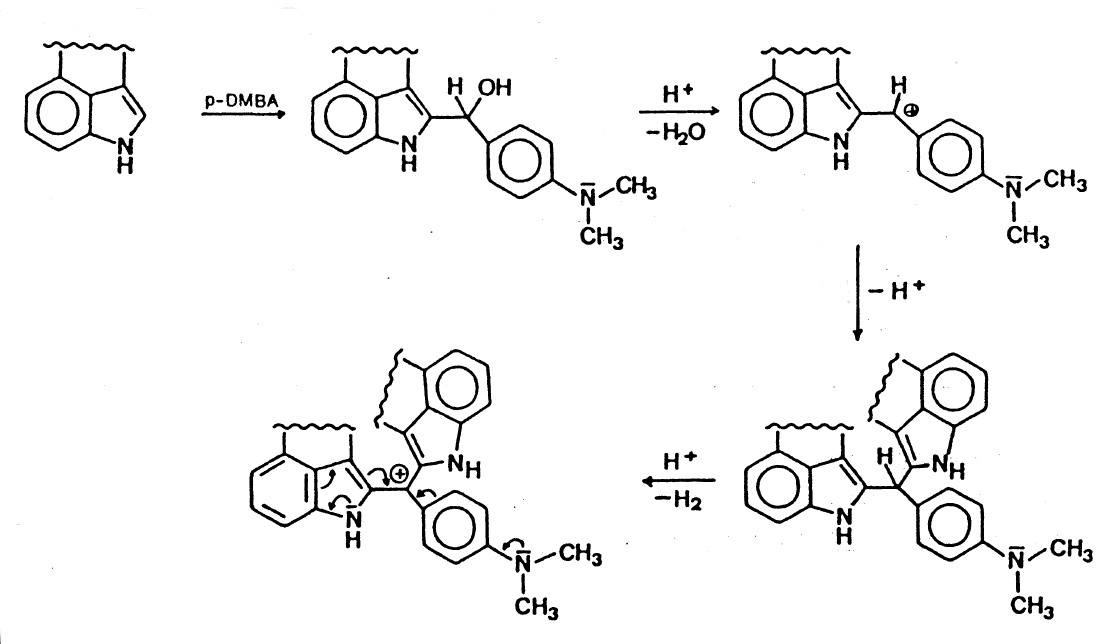
Ehrlich's Reagent and reactions with indole type derivatives like
LSD
The colouring matter produced is determined by the concentration
of the acid, the solvents and other conditions of the reaction.
After the condensation of one molecule of LSD with one molecule of
p-dimethylamino benzaldehyde, the carbinole is formed. After
protonation water is eliminated to form the carbenium ion, which
then reacts by the addition of a second molecule of LSD. This is
finally oxidized to the blue-coloured cyanine.
Duquenois-Levine Reagent
The test was initially developed in the 1930s by Pierre Duquenois,
and was adopted in the 1950s by the United Nations as the preferred test
for cannabis, and originally claimed to be specific to cannabis.
The reagent can be prepared by adding 2 grams of vanillin and 2.5 milliliters
of acetaldehyde to 100 milliliters of ethanol.
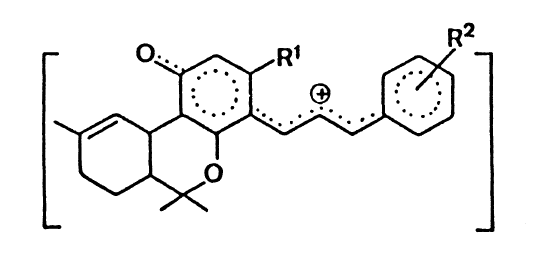

Duquenois-Levine Reagent
The Duguenois-Levine Test describes the determination of cannabis
resin by forming the violet-coloured product above, which can be extracted
with chloroform.
It is now recognised that it is NOT specific however, despite this, it may
be the
only test performed on substances and be used as evidence for conviction.
KN (Fast Blue B Salt) Reagent
KN= Kanto-Shin'etsu Narcotics Control Office, Japan
Presumptive test designed to identify the presence of THC in Marijuana,
Hashish or Hash Oil. It is also designed to react with the green leaf material
of fresh Marijuana. Upon breakage of the 2nd ampoule, a layering will occur
and the lower layer must be a Tomato Red colour for a positive test.
The Reagent contains a solution of naphthanil diazo blue B in chloroform and a
solution of aqueous sodium hydroxide.
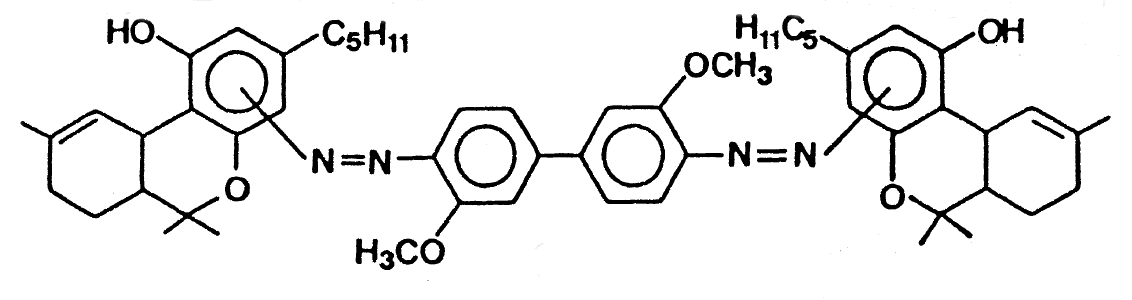
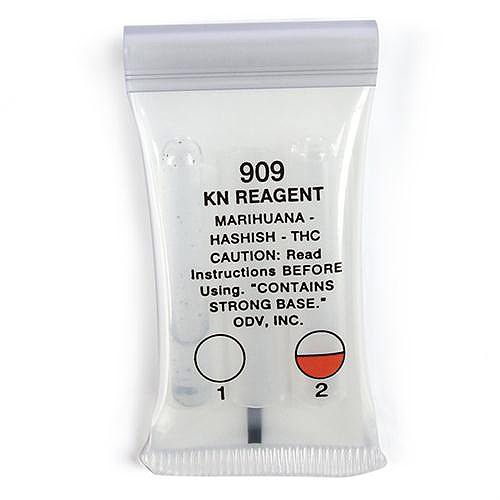
Possible product of Cannabis detection with Fast Blue B
Valium Reagent - Zimmerman Test
A solution of potassium hydroxide in methanol and a solution of
m-dinitrobenzene in isopropanol.
Diazepam,
more commonly known by its trade name, Valium, is a
benzodiazepine and a controlled substance (Schedule IV in the
United States and under International Control as well). It is a potent
sedative - hypnotic (CNS
depressant), and is one of the most prescribed drugs in the
world. It is one of the top five most abused
benzodiazepines, and
misuse can lead to both psychological dependence and/or physical
addiction.
The State of California offers diazepam to condemned inmates as a
pre-execution sedative as part of their Lethal Injection program.
Drug-facilitated sexual assault - Date-rape
drugs
Flunitrazepam Rohypnol,
Rufinol or Roofies are known to induce anterograde amnesia in sufficient doses;
individuals are unable to remember certain events that they
experienced while under the influence of the drug. This effect is
particularly dangerous when flunitrazepam is used to aid in the
commission of sexual assault; victims may be unable to clearly
recall the assault, the assailant, or the events surrounding the
assault.
Rohypnol is manufactured by Hoffman-La Roche. It is sold in tablet form in
clear bubble packaging, with the tablets inscribed on one side with the
word "Roche." It has no taste or smell. Originally the tablets were
manufactured only in white and, if dissolved in liquid, did not affect
its colour. But once it became known that Rohypnol was being used to
facilitate rape, Hoffman-La Roche began manufacturing an additional
version coated in a hard green shell to inhibit dissolution, and
containing a strong blue dye.
It is difficult to estimate just how many
flunitrazepam-facilitated rapes have occurred.
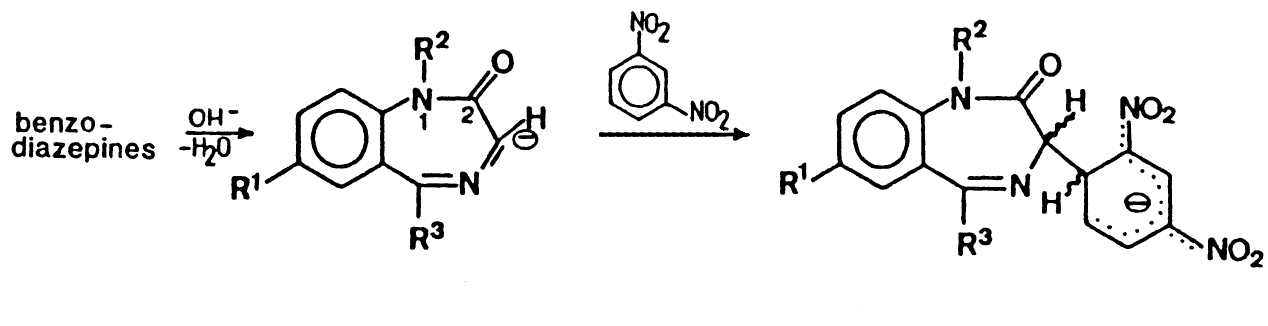
Zimmerman's test for Benzodiazepines
Methamphetamine, Simon's and Robadope's Reagent
Simon's reagent is composed of an aqueous mixture of sodium nitroprusside,
sodium carbonate and acetaldehyde, which is dripped onto the substance
being tested. The amine and acetaldehyde produce the enamine, which
subsequently reacts with sodium nitroprusside to the imine. Finally, the
immonium salt is hydrolysed to the bright cobalt-blue Simon-Awe complex.
Robadope reagent can be used for detecting primary amines.
Acetaldehyde can be replaced with acetone, in which case the reagent detects
primary amines instead, giving a purple coloured product.
Note the paper for identification of methamphetamines using FTIR that was
recently published in PLoS ONE:
Hughes J, Ayoko G, Collett S, Golding G (2013)
Rapid Quantification of Methamphetamine: Using Attenuated Total Reflectance
Fourier Transform Infrared Spectroscopy (ATR-FTIR) and Chemometrics.
PLoS ONE 8(7): e69609. doi:10.1371/journal.pone.0069609
Mecke's Modified Reagent
The Mecke reagent is used as a simple spot-test to presumptively identify
alkaloids as well as other compounds. The reagent which is prepared by the
addition of 100 mL of concentrated sulfuric acid to 1 g of selenious acid
is dripped onto the substance being tested.
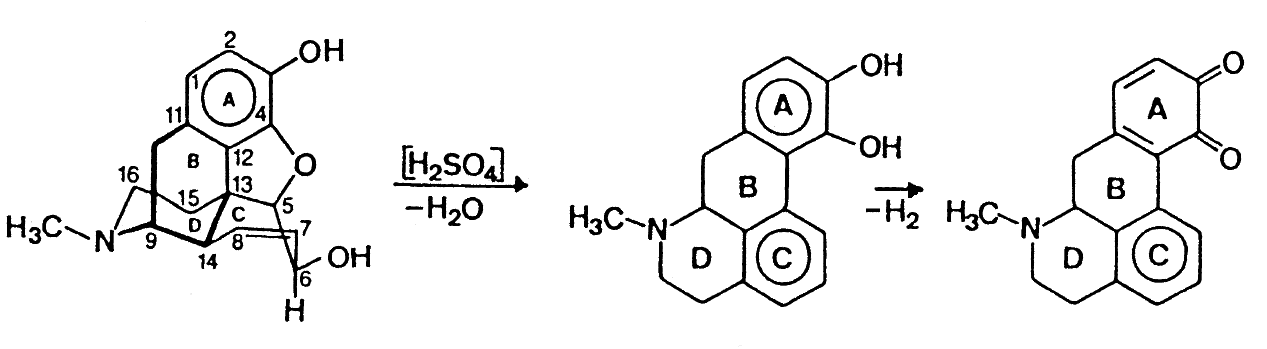
Mecke's Reagent reaction with morphine
The blue to green colour produced by Mecke's Reagent with
morphine is thought to arise from the initial rearrangement to
apomorphine, which in the presence of selenious acid is oxidized
to the o-quinone of apomorphine.
Frohdes Talwin Reagent
Frohdes Reagent - Presumptive test designed to identify the
presence of
Pentazocine
which is a synthetically prepared prototypical mixed narcotic
(opioid analgesic) drug of the benzomorphan class of opioids used to treat
moderate to moderately severe pain. It may exist as one of two enantiomers,
(+)-pentazocine and (-)-pentazocine. (-)-pentazocine is a κ opioid
receptor agonist, while (+)-pentazocine is not.
After the discovery of its misuse, naloxone was added to oral preparations
containing pentazocine to prevent the opioid reaction.
Upon breakage of the 2nd ampoule and after shaking, a bright blue appears
in the presence of Pentazocine.
The Reagent is prepared from 1 g of sodium molybdate in 100 mL of
concentrated H2SO4.
Ephedrine Reagent
An aqueous solution of sodium carbonate and sodium
nitroferricyanide and a solution of m-dinitrobenzene in
isopropanol.
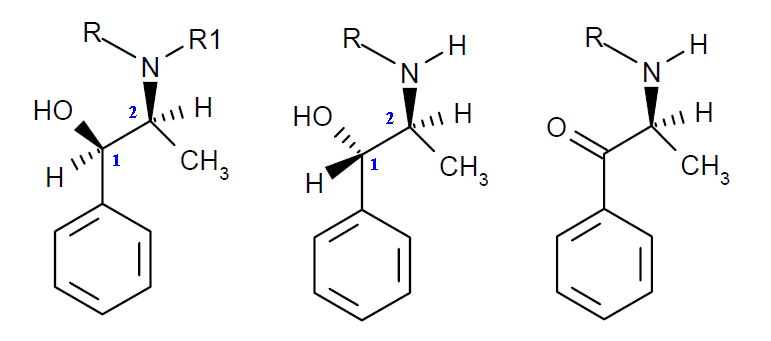
(1R,2S)(-)-Ephedrine, (1S,2S)(+)-(pseudo-ephedrine and related species and an Ephedrine
Reagent Kit
Detection of
ephedrine by its reaction with copper sulfate and sodium hydroxide,
was first noted by Nagai (1892): a purple color appears,
which is extractible with ether. This test is sensitive to one part of
ephedrine in 400, and if the concentration exceeds 1 in 40 a pinkish
purple precipitate is formed, and this is completely soluble in ether.
The formation of a violet-coloured chelate complex with copper
sulfate in alkaline medium is considered to be characteristic
"selective" for phenylalkylamines with vicinal amino- and
hydroxyl-groups and has been in use in pharmaceutical analysis
for the simple identification of
ephedrine,
pseudoephedrine,
norephedrine and norpseudoephedrine.
The result of the Chen-Kao
reaction is a symmetrical chelate complex. The
colour, solubility and the stability of this complex appears to
be affected by the structural and steric differences of the
alkylamine. Tests on a range of ephedrine-type compounds showed
that only ephedrine and pseudoephedrine gave the typical violet
solution. Other derivatives produced a blue precipitate.
|
Cu(ephedrine)2 complex, Bull. Chem. Soc.,
Jpn., (1964), 37, 1363,
|
Special Opiates
A solution of sulfuric acid and selenious acid.
PCP Reagent
An aqueous solution of cobalt thiocyanate and concentrated
phosphoric acid.
References
UWI students can see OurVLE for copies of some of the reports mentioned above.
Kovar,
Karl-Artur and Martina Laudszun. Chemistry and Reaction
Mechanisms of Rapid Tests for Drugs of Abuse and Precursors
Chemicals, United Nations Scientific and Technical Notes
v.89-51669, Germany. February 1989.
National
Institute of Justice. Colour Test Reagents/Kits for Preliminary
Identification of Drugs of Abuse, NIJ Standard 0604.01, National
Law Enforcement and Corrections Technology Center, Rockville, MD.
July 2000.
United Nations Office On Drugs and Crime (UNODC), Laboratory and
forensic science services
SCIENTIFIC
AND TECHNICAL NOTES SCITEC/20 December 2005 Colour Tests for
Precursor Chemicals of Amphetamine-Type Substances
United Nations International Drug Control Programme. Rapid
Testing Methods of Drugs of Abuse, UN standard ST/ NAR/13/RE V.1,
United Nations, New York, NY. 1994.
Continue to GC-IRMS or return to CHEM2402 course outline.

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica
Created October 2011. Links checked and/or last
modified 7th November 2014.
URL
http://wwwchem.uwimona.edu.jm/courses/CHEM2402/Crime/Reagent_KitsJS.html
























 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,