Department of Chemistry
University of the West Indies, Mona,
JAMAICA
 |
HEXOL |
| By Prof.
Robert J. Lancashire
Department of Chemistry
|
Later that year, Werner was appointed at the University of Zurich to teach organic chemistry and subsequently 45 of his 174 publications dealt with the organic chemistry of oximes, hydroxamic acids, and dyestuffs etc. In fact, it was not until 1902-1903 that he started to teach the main lecture course in inorganic chemistry.
During the period 1893 until his death in 1919, he directed numerous dissertations (some 200 altogether, 56 of these between 1894 and 1902) and maintained a vibrant laboratory which must obviously have benefitted from collaborators from countries as diverse as Japan, Italy, UK, Sweden, the USA, and the USSR.
In 1913, he received the Nobel prize the first in Inorganic Chemistry. He was chosen "in recognition of his work on the linkage of atoms in molecules, by which he has thrown fresh light on old problems and opened new fields of research, particularly in inorganic chemistry."

A consequence of the octahedral model was that optical isomers should be possible for certain types of complexes. It is not clear when Werner actually realised this since it was not until 1897, that is 4 years after the original proposal, that he mentioned in a letter to Arturo Miolati that he was searching for optically active complexes. (It was with Miolati that he had published his first experimental work on coordination complexes, dealing with molecular conductance.(4))
In June 1911, when Victor King successfully resolved cis-[CoCl(en)2NH3]2+, Werner skipped an evening lecture, a most unusual event. He was apparently worried that the sample would racemise overnight and wanted to prepare derivatives and collect as much information as quickly as possible. As it happened, the samples were quite stable.
Within eight years, Werner and his students had gone on to resolve another 40 complexes. However, because of the prevailing view that optical activity was integrally related to C atoms, Werner decided that it was necessary to prepare a completely inorganic complex (i.e., no C atoms) to prove that "carbon free inorganic compounds can also exist as mirror-image isomers".
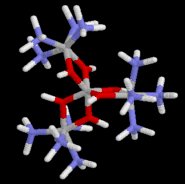
Werner's communication dealing with the resolution and optical activity of hexol as the bromide salts (tris-{tetraammine-u-dihydroxo-cobalt(III)}cobalt(III) bromide dihydrate) was published in 1914. It had taken him 11 years from the original proposal of the octahedral model to amass sufficient experimental evidence finally to silence his opponents.
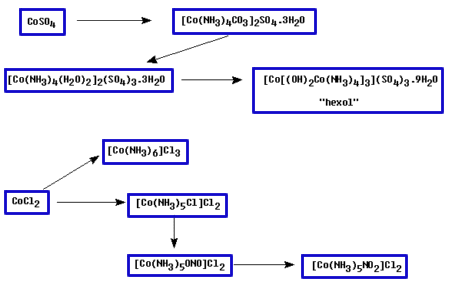
The starting material for his scheme was either Hexol sulfate, which he converted to the chloride by reacting with barium chloride, or Hexol chloride obtained from [CoCl(NH3)4(H2O)]Cl2. He then treated this Hexol chloride salt with the resolving agent, silver d(+)-bromocamphorsulfonate, in dilute acetic acid.
The less soluble species (first precipitate) was found to be the d-Hexol salt which was then converted to the bromide. From the filtrate, the l-isomer could be isolated and converted to the bromide as well.
More recently, higher optical purity of the isomers has been achieved by using sodium bis(u-d-tartrato)-diantimonate(III) as the resolving agent. A laboratory experiment, suitable for an undergraduate inorganic course, has been published based on the revised scheme.(5)
Another laboratory exercise that gives a fairly simple sequence to the racemic mixture of hexol sulfate, was published in 1982(6). The reaction scheme in this case is given below.
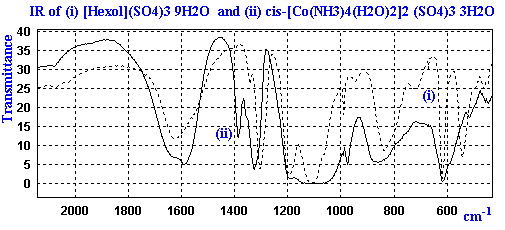
The IR of these complexes give fairly broad bands due to the presence of the water, ammonia, and sulfate ions. An expanded section of the IR for the diaqua and hexol salts is shown below as a bitmap.
If you have JavaScript enabled in your browser, then JSmol can be used to
view the "live" spectra of the complexes, using the links below:
1) [CoCO3(NH3)4]2 SO4.3H2O IR and Vis
2) cis-[Co(NH3)4(H 2O)2]2 (SO4)3 .3H2O
3) [Co[Co(OH)2(NH3) 4]3] (SO4)3 .9H2O - hexol sulfate enneahydrate
Since the resolution of hexol, only a handful of other "completely inorganic complexes" have been resolved, including one, (NH4)2[PtS15] which was once described in the title of the paper as "stinking rich" (7).
A study here at Mona(8) was concerned
with determining which of two mechanisms was involved in the rate
determining step of the reduction of hexol by L-ascorbic acid.
Did the reaction occur at the more accessible outer cobalt ions
or at the central cobalt ion? The results suggest that it is
probably at the central cobalt ion.
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
© 1997-2021 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,