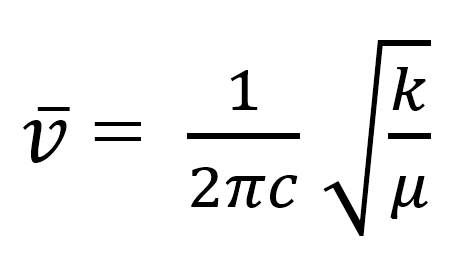
is derived from Hooke's law, where ν is in wavenumbers, k = the force constant,
and μ is the reduced mass of the system:
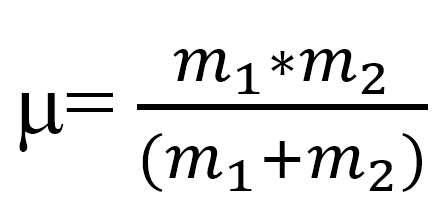
|
Simulation of spectra using Gaussian (WebMO.net) IR CH2Cl2 ---- R-- CH2Cl2 ---- overlay the two --- split overlay IR CHCl3 ---- R-- CHCl3 ---- overlay the two --- split overlay IR of Liquid samples CH2Cl2 ---- CD2Cl2 ---- overlay the two --- split overlay CHCl3 ---- CDCl3 ---- overlay the two --- split overlay |
Toggle Grid Toggle Coordinates Reverse plot |
|
|
Character table for C2v point group
|
Character table for C3v point group
|
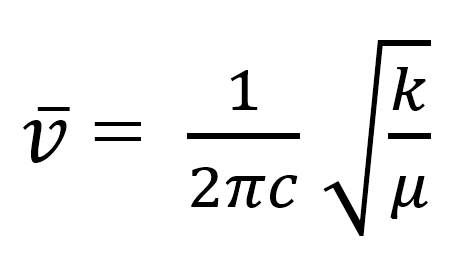
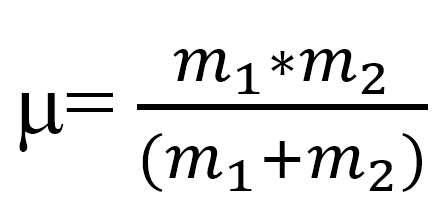
Copyright © 2019 by The Department of Chemistry, UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,