Experiment 12
Preparation of 4-acetoxybenzoic acid
Experimental Aims The aim of this exercise is to synthesise a
phenyl ester (using acid catalysis), recrystallise the product,
and determine its purity using melting point data and thin layer
chromatography.
Experimental learning objectives At the end of
this experiment you should be able to: (i) precipitate an organic
compound from a reaction mixture by addition of water; (ii)
isolate a solid by filtration; (iii) recrystallise a compound
from ethanol-water; (iv) determine melting points; (v) calculate
the yield of a product; (vi) perform chemical tests for phenols
and esters; (vii) establish the purity of a compound by tlc;
(viii) determine the relative polarity of starting material and
product using tlc; (ix) calculate Rf values.
Introduction Phenols, unlike amines, cannot be acetylated
satisfactorily in aqueous solution: acetylation of phenol proceeds readily
with acetic anhydride in the presence of a little concentrated
sulfuric acid as catalyst. In this experiment you will synthesise
a compound, purify it by recrystallisation, and determine its
melting point.
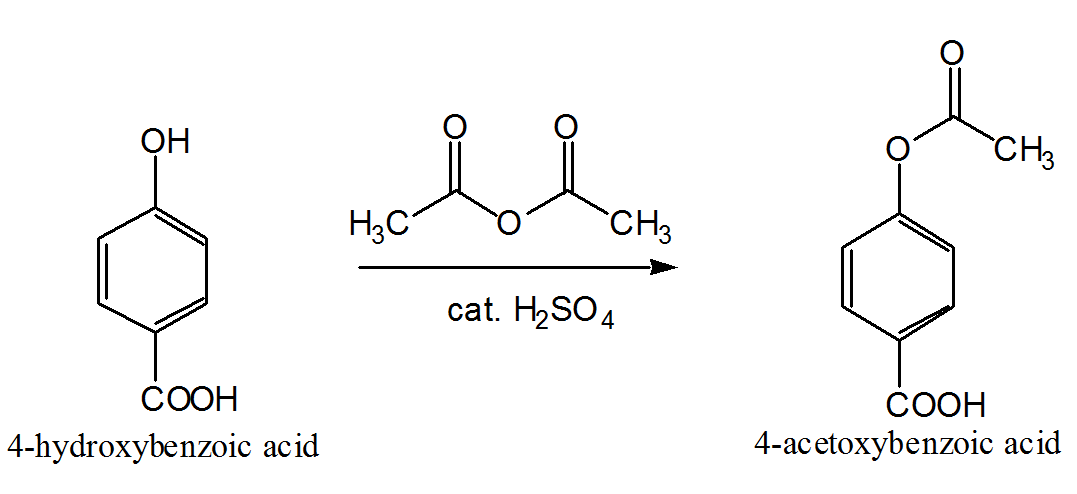
Mechanism
Procedure Place 2 g. of dry 4-hydroxybenzoic acid and
3 g (3 mL) of acetic anhydride (Note 1) in a small
conical flask, add 1 drop of concentrated sulfuric acid, and rotate the flask
in order to secure thorough mixing. Warm on a water bath to about
50-60ºC, with stirring, for about 15 minutes. Allow the
mixture to cool and stir occasionally. Add 30 mL of water, stir
well and filter the precipitate at the pump.
Dissolve the solid in 6 mL of hot ethanol (NO FLAMES!) and pour
the solution into 15 mL of warm water. If a solid separates at
this point, warm the mixture until it dissolves and then allow
the clear solution to cool slowly. Beautiful needle-like crystals
will separate (be careful not to heat your solution too strongly
because some decomposition of your product may occur).
Determine the melting point (Note 2) and
calculate the yield of the product.
Perform the following tests on the starting material
(appendix 1):
(i) bromine water test;
(ii) ferric chloride test.
Perform the following tests on your product
(appendix 1):
(i) hydroxamic acid test;
(ii) bicarbonate test.
Note 1: Acetic (ethanoic) anhydride is LACHRYMATORY and should be handled with care under a
FUME HOOD.
Note 2: The m.p. of this compound is between 150
and 200 ºC
Use of thin layer chromatography (tlc) as an
analytical technique
Objective: To show how TLC may be
used to assess the purity of a compound and to determine the
components present in a mixture.
Introduction:
The term chromatography describes a technique whereby substances
may be separated from one another when they are partitioned
between two phases, a mobile phase and a stationary phase.
Suppose a mixture of two compounds A and B is placed, for
example, on a column of silica (the stationary phase) and that B
is more strongly adsorbed than A. If a liquid (the mobile phase,
in which both compounds are soluble) is now passed over the
stationary phase both A and B will tend to be removed from the
silica and be carried along in the direction of liquid flow.
Since B is more strongly adsorbed than A on the silica, it is
less easily removed by the liquid. If the latter is collected in
fractions, it will be observed that the first fraction will
contain compound A only and the latter fractions will contain
compound B. The original mixture is thus separated into its
individual components.
The above separation technique was first applied to the
separation of coloured compounds, e.g. the separation of pigments
in plant material, but it is now widely used for both coloured
and non-coloured materials. The following types of
chromatographic separation are routinely employed in chemical
laboratories:
1. Column
2. Paper
3. Thin layer
4. Gas-solid and gas-liquid chromatography.
In thin layer chromatography, the stationary phase (e.g. silica,
alumina, cellulose) is deposited as a thin layer (0.1 - 2 mm
thick) on a flat supporting surface, normally a piece of glass or
polyester of suitable dimensions (e.g. 5 cm x 20 cm x 0.5 cm).
The adsorbent is generally held in place with a binding agent
such as starch or plaster of Paris. The mixture to be separated
is first dissolved in a suitable solvent, then applied (by means
of capillary) as a small spot on the stationary phase a short
distance from one end. The plate is then placed vertically in a
developing chamber containing a small amount of a suitable
solvent, which serves as the mobile phase. The latter should be
sufficient to cover the lower edge of the plate but the liquid
surface must be below the applied spot. The chamber is closed and
the solvent is allowed to ascend the layer by capillary action
until it is a short distance from the upper edge of the plate.
The latter is then removed from the chamber and the height of the
solvent front noted. If the experimental conditions are carefully
selected, the components in the mixture will be resolved as
separate spots. If the components are coloured compounds they may
be seen directly, or if colourless, they may be made visible by
exposure to iodine vapour or by viewing the plate under
ultraviolet light (if the layer contains a fluorescent
indicator).
The behaviour of a particular component in a specific
chromatographic system is frequently described by its
Rf value. This is derived by means of the
equation:
Rf = distance travelled by compound / distance
travelled by solvent
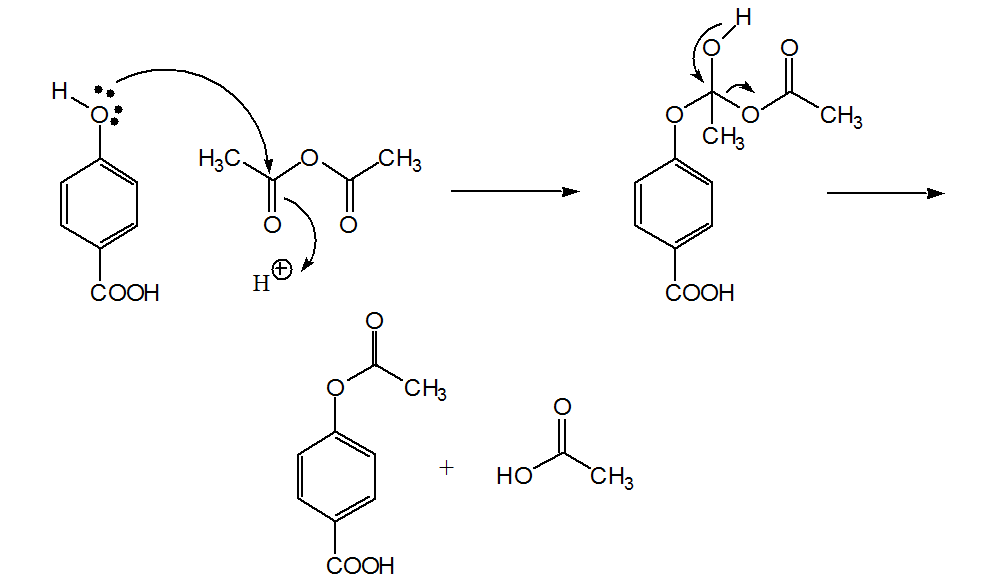
Procedure:
Dissolve a few crystals of the starting material
(4-hydroxybenzoic acid) provided in the minimum amount of
ethanol. Use a capillary to place a small spot (not more than 3
mm in diameter) on the left side of the TLC plate about 0.5 cm
from the bottom (Note 1). Allow the spot to dry in air. Repeat
with your ester (4-acetoxybenzoic acid), placing the spot on the
right side of the plate. You now have two on spots the same
plate. Measure approximately 8 mL of the developing solvent (15
parts toluene to one part acetone) and transfer it to a clean,
dry 250 mL beaker. The liquid level should be no higher than 0.5
cm. Place the TLC plate to stand in the beaker with the top end
resting on the wall of the beaker and cover it with a watch
glass. Allow the solvent to rise within 1 cm of the top edge of
the plate, keeping the beaker covered. Remove the plate from the
beaker, and allow it to dry in air after marking the position of
the solvent front (Note 2). Observe the plate under an
ultraviolet lamp and mark with a pencil the position of any
visible spots.
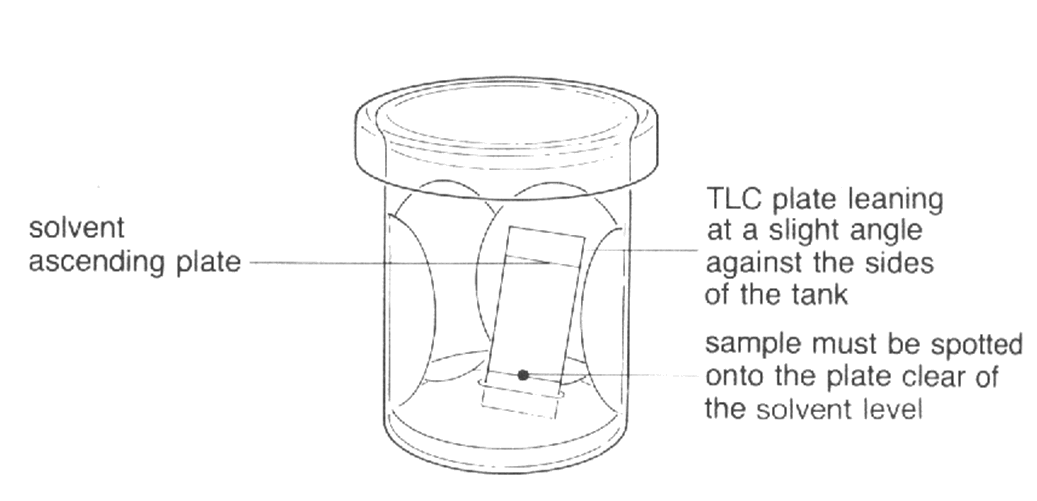
Record the number of components present in each sample and obtain
the Rf (retention factor) value for each component.
Show the TLC plate to your demonstrator. Do not paste it in your lab work sheet.
Note 1: If the spot applied is too large, it
becomes diffuse as it is carried along by the liquid phase and
the components may not be resolved satisfactorily.
Note 2: Discard
eluant in waste bottle provided and not down the
sink.
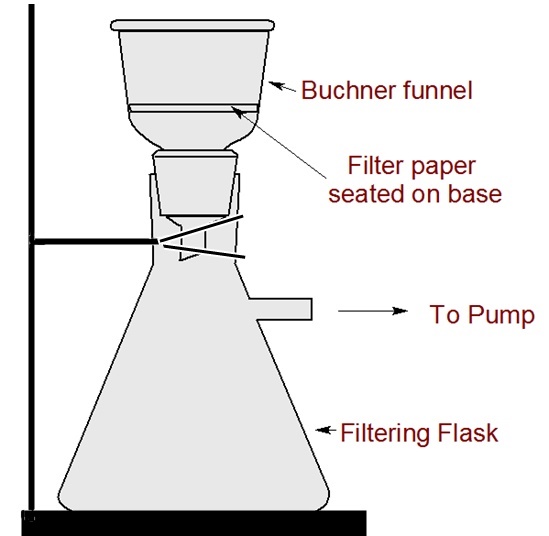
Filtering Apparatus
The IR and vibrational modes of 4-hydroxybenzoic acid and 4-acetoxybenzoic acid
are shown as an example of an interpreted spectrum using a non-Java
interactive JSmol display (HTML5/JavaScript)
Note: The spectrum of the 4-hydroxy species was recorded for the gas phase,
not condensed phase.
Load
4-OH,
4-Ac,
Overlay them
grid off
grid on
show peak list
Acknowledgements
Jmol/JSpecView code conversion to JavaScript by Bob Hanson.
Copyright © 2011-2014 by The Department of Chemistry
UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Oct 2011. Links checked and/or last
modified 29th October 2014.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c1901exp12.html




