| Property | OF2 | O2F2 | Cl2O | ClO2 | I2O5 |
|---|---|---|---|---|---|
| Physical appearance and general characteristics | Colourless (very pale yellow) gas; explosive and toxic | Yellow solid below 119 K; decomposes above 223 K | Brownish yellow gas; explosive at high concentrations | Yellowish gas or liquid; explosive above 15% volume in air | white crystalline hygroscopic solid |
| Melting point /K | 49 | 119 | 153 | 213 | 573 decomp |
| Boiling point /K | 128 | 210 | 275 | 284 | - |
| ΔfH°(298 K) / kJ mol-1 | 24.7 | 18 | 80.3 | 102.6 | -158.1 |
| Dipole moment /D | 0.3 | 1.44 | 0.78 | 1.78 | - |
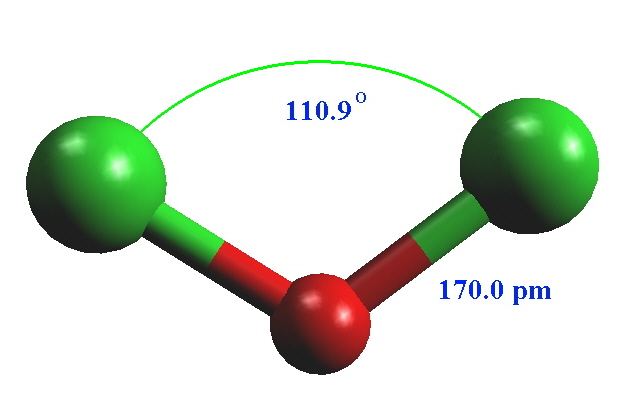
| O-X bond distance /pm | 141 | 157.5 | 170.0 | 147.3 | 180 (term) 195 (bridging) |
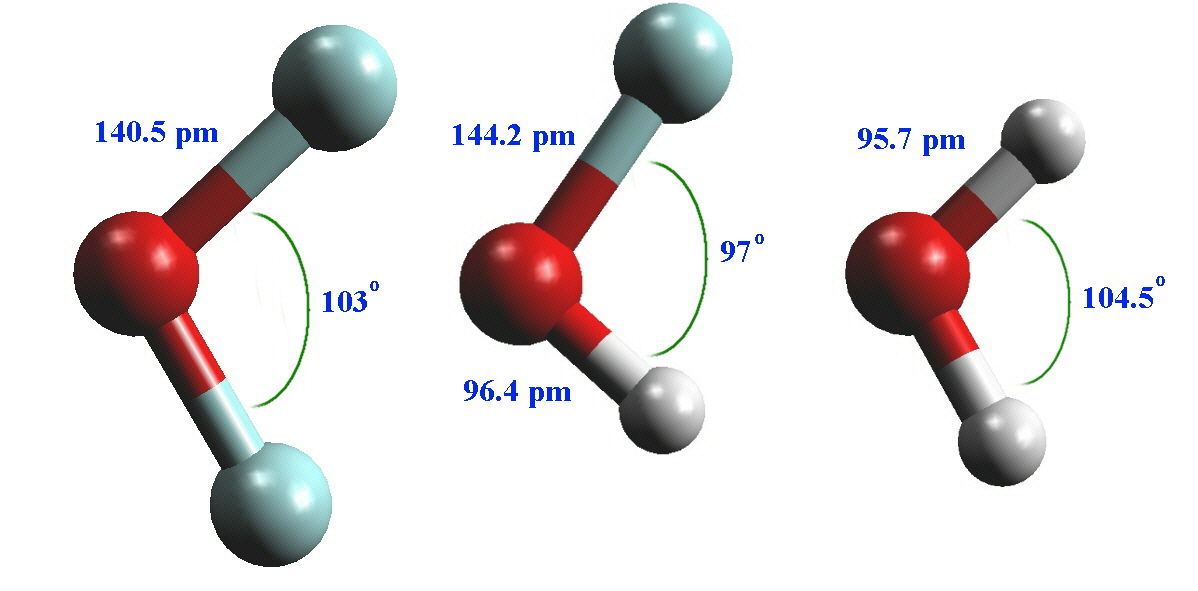
The shape of the OF2 molecule has been compared with HOF and H2O and the three give bent structures where the bond angles range from 97 to 104.5 °.

Cl2O is best prepared by treating fresh yellow
mercuric oxide with Cl2 gas:
2 Cl2 + 2 HgO → Cl2O + HgCl2·HgO
It can also be prepared by reaction of Cl2 gas with
moist sodium carbonate, Na2CO3.
2Cl2 + 2Na2CO3 + H2O
→ Cl2O + 2NaCl + 2NaHCO3
Dichlorine monoxide is very water soluble (a saturated solution
at -9.4 °C contains 143.6 g Cl2O per 100 g
H2O) and it hydrolyses to hypochlorous acid.
Cl2O + H2O → 2HOCl
Chlorine dioxide (ClO2) was mentioned in the first
lecture as being an important industrial chemical for bleaching
flour and wood pulp and for water treatment. Over 95% of the
chlorine dioxide produced in the world today is made from sodium
chlorate:
2NaClO3 + 2NaCl + 2H2SO4 →
2ClO2 + Cl2 + 2Na2SO4
+ 2H2O
ClO2 was first discovered by Humphry Davy (1778-1829) in 1811 and was the first of the chlorine oxides to have been isolated. The yellow paramagnetic gas explosively decomposes into chlorine and oxygen and the decomposition is initiated by light. Thus, it is never handled in concentrated form, but is almost always used as a dissolved gas in water in a concentration range of 0.5 to 10 grams per liter. Its solubility increases at lower temperatures: it is thus common to use chilled water (5 °C) when storing at concentrations above 3 grams per liter. In many countries, such as the USA, chlorine dioxide gas may not be transported at any concentration and is almost always produced at the application site using a chlorine dioxide generator. Chlorine dioxide is less corrosive than chlorine and superior for the control of legionella bacteria. It is more effective as a disinfectant than chlorine in most circumstances against water borne pathogenic microbes such as viruses, bacteria and protozoa.
The oxidation number of the Cl is considered to be +3 and Pauling originally proposed that resonance structures be used to account for this. An alternative explanation by Brockway extended this to resonance between 3 electron 2 centre bonds. For many years it was claimed that a dimer was not formed and considering that ClO2 is a radical this might have been one mechanism to provide some stability by using up the odd electron in a 2 electron 2 centre bond. In 1992, Rehr and Jansen conducted magnetic susceptibility and single crystal studies at various temperatures and it was found that below about 170 K the sample became diamagnetic and did indeed form a dimer. They noted that to avoid explosions all equipment had to be scrupulously cleaned with aqua regia prior to use and temperatures kept low.
The oxides of bromine are less numerous than for chlorine and they are generally unstable at room temperature decomposing to the elements.
For example, BrO2 is a pale yellow crystalline solid formed quantitatively by low-temperature ozonolysis of dibromine.The oxides of iodine are the most stable of the halogens and the first of these to be prepared was I2O5 which was independently discovered by Joseph Louis Gay-Lussac (1778-1850) and H. Davy in 1813 although the structure was not determined until 1970. The white hygroscopic crystals are very soluble in water and commercial I2O5 has been found to consist of HI3O8 or I2O5.HIO3. Likewise crystals of commercial iodic(V) acid, HIO3, were investigated using single-crystal and powder X-ray diffraction and the crystals turned out again to be HI3O8 or HIO3·I2O5.
I2O5 is one of only a few chemicals that
is capable of oxidising carbon monoxide rapidly and completely at
room temperature:
I2O5 + 5 CO → I2 + 5
CO2
This reaction has been used as the basis of an analytical method
for sampling CO in the atmosphere. I2O5 can
be used for the oxidation of a number of other small molecules
including: NO, C2H4, SO3,
H2S.
Numerous oxoacids of the halogens exist and chlorine is able to form a complete series of such acids ranging from HOCl to HClO4 where the formal oxidation state is +1,+3,+5 and +7. [1]
| Oxoacids of chlorine | Oxoacids of bromine | Oxoacids of iodine |
|---|---|---|
| Hypochlorous acid HOCl, pKa=7.53 | Hypobromous acid HOBr, pKa=8.69 | Hypoiodous acid HOI, pKa=10.64 |
| Chlorous acid HOClO (HClO2), pKa=2.0 | ||
| Chloric acid HOClO2 (HClO3), pka=-1.0 | Bromic acid HOBrO2 (HBrO3) | Iodic acid HOIO2 (HIO3) |
| Perchloric acid HOClO3 (HClO4), pka=~-8 | Perbromic acid HOBrO3 (HBrO4) | Periodic acid HOIO3 (HIO4) |
| Orthoperiodic acid (HO)5IO (H5IO6) |
HOF has been isolated by reaction of difluorine gas with ice at
230 K but it does not ionise in water or give stable salts and on
warming to room temperature it decomposes to HF.
2 HOF → 2 HF + O2
The hypohalous salts formed from the heavier halogens are all
weak acids with the hypochlorites being important industrial
reagents.
Bleaching powder is a mixture of CaCl2,
Ca(OH)2 and Ca(OCl)2 and is manufactured by
the reaction of dichlorine on Ca(OH)2. The sodium salt
is a well known disinfectant and bleaching agent
Chlorous acid, chloric acid and bromic acid cannot be isolated
as pure compounds but exist in aqueous solution and are prepared
from the barium salts.
Ba(ClO2)2 + H2SO4(aq)
→ 2 HClO2(aq) + BaSO4(s)
and Ba(XO3)2 + H2SO4
→ 2 HXO3 + BaSO4 (X= Cl, Br)
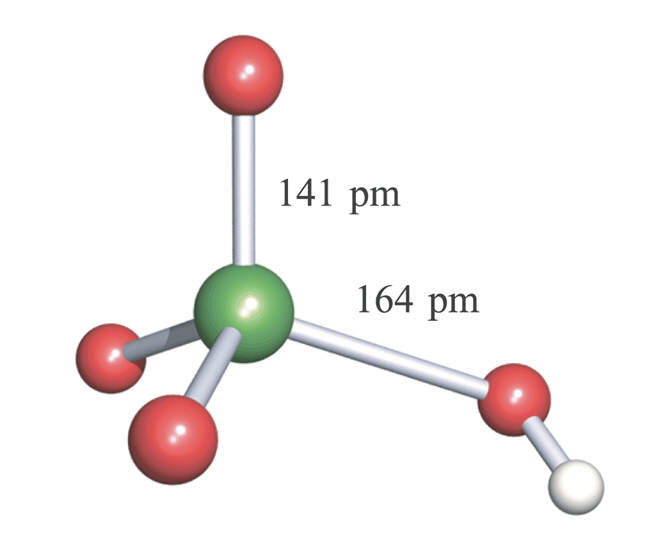
The structure below shows trigonal pyramidal HIO3 units and these are connected by extensive H-bonding. In aqueous solution it is a reasonably strong acid with pKa ~ 0.77.
The materials used need to be of high purity since even small amounts of impurities can lead to explosions and it is considered good practice to test a new batch of potassium chlorate with a small reaction first (less than 1 g!) Even commercial oxygen generators have been known to explode and cause problems such as at least one plane crash and a fire on the space station MIR.[10]
Both potassium bromate and iodate are used in volumetric analysis and in
particular the iodate is a primary standard being available in high purity
and with excellent stablity. In the standardisation of thiosulfate for example
potassium iodate is used a source of diiodine.
[IO3]- + 5 I- + 6 H+ → 2 I2 + 3 H2O
Iodate is used in Andrew's titrations for determination of hydrazine and
in the determination of I- in SnI4 in the CHEM3101 laboratory programme.
|
HIO3 |
H5IO6 orthoperiodic acid |
Several different I(VII) oxyacids are known including periodic acid (HIO4) and
orthoperiodic acid (H5IO6). In the latter case there is
extensive H-bonding present in the solid state resulting in a 3D network.
Wherease perchloric acid exists as discrete monomeric units HIO4 is again
extensively H-bonded through cis- edge-sharing octahedra.
The reactions involving the various oxyacids in aqueous solution is
complicated, as seen by the following equilibria:
[H3IO6]2- + H+ → [IO4]- + 2H2O
2[H3IO6]2- → 2[HIO5]2- + 2H2O
2[HIO5]2- → [H2I2O10]4- → [I2O9]4- + H2O
Count Friederich von Stadion is credited with the preparation of the first perchlorate compound in 1816 which he obtained by mixing potassium chlorate with concentrated sulfuric acid and allowing it to stand for 24 hours with frequent agitation. The water-insoluble residue was potassium perchlorate. Perchloric acid is the only oxoacid of chlorine that can be isolated and in the vapour phase the Cl-O bonds are not equivalent unless deprotonated when they become the same length.
Texas chemists have shown evidence for a natural atmospheric origin of perchlorate. For many, perchlorate is almost synonymous with the rocket fuel that has found its way into U.S. groundwater and surface water and subsequently into foods such as lettuce and milk. Perchlorate taints drinking water in 35 US states at levels of at least 4 ppb. When groundwaters of a 60,000-sq-mile region in Texas and New Mexico were sampled, > 80% of the wells returned positive and unexpectedly high levels of perchlorate and it was found even in samples that were unlikely ever to have been exposed to synthetic contamination. The perchlorate levels in many of the Texas samples were more than 20 ppb, and some were as high as 60 ppb. However, there were regions such as the southern high plains (Texas Panhandle) where there was no clear historical or current evidence of the extensive presence of rocket fuel or Chilean fertilizer sources. The occurrence of easily measurable concentrations of perchlorate in such places was difficult to understand. In the southern high plains groundwater, perchlorate was better correlated with iodate, known to be of atmospheric origin, compared to any other species. They showed that perchlorate was readily formed by a variety of simulated atmospheric processes. For example, it could be formed from chloride aerosol by electrical discharge and by exposing aqueous chloride to high concentrations of ozone. They further reported that perchlorate was present in many rain and snow samples, strongly suggesting that some perchlorate was formed in the atmosphere and a natural perchlorate background of atmospheric origin existed.[4][5][6]
Although the scientists were fairly certain that the perchlorate in the Texas wells was of natural origin, they still needed more evidence to prove it. Researchers at Louisiana State University (LSU) and Oak Ridge National Laboratory (ORNL) developed a new stable-isotope ratio technique that could provide the missing piece of information.
To distinguish between natural and synthetic perchlorate it is important to recognise that perchlorate is a non-volatile oxyanion and that once formed it does not exchange oxygen atoms with those in the ambient environment. This means its oxygen ratio signature is fixed. So by looking at both the 17O/16O and 18O/16O ratios it is possible to tell whether it is from a natural source or not.
In this approach, all three stable oxygen isotopes are measured after a thermal decomposition method generates O2 from perchlorate crystals. Measuring just 18O/16O won't give you the answer, because 18O/16O ratios for anthropogenic and atmospheric perchlorate overlap. But if you measure 17O/16O at the same time and compare the two ratios, it is possible to determine its origin. Most oxygen-containing compounds on earth have a correlation between 18O/16O and 17O/16O. Any deviation from this relationship is referred to as the 17O anomaly, or Δ17O. The results of studies on Chilean perchlorates from the Atacama desert caliche deposits found that Δ17O was between 4 and 10 whereas for man-made perchlorates the value was generally less than -0.2.[7]
In the human body, perchlorate inhibits the uptake of iodide by the thyroid and thus may lower the amount of thyroid hormone in the body. Insufficient levels of this hormone can cause permanent neurological damage in children.
The changes in perchlorate concentrations in surface water adjacent to a site of fireworks displays from 2004 to 2006 was monitored. Preceding the fireworks displays, perchlorate concentrations in surface water ranged from 0.005 to 0.081 µg/L, with a mean value of 0.043 µg/L. Within 14 h after the fireworks, perchlorate concentrations spiked to values ranging from 24 to 1028 times the mean baseline value. A maximum perchlorate concentration of 44.2 µg/L was determined following the July 4th event in 2006. After the fireworks displays, perchlorate concentrations decreased toward the background level within 20 to 80 days, with the rate of attenuation correlating to surface water temperature.[8]
The Pentagon, NASA, the US Department of Energy, and defense contractors - who could face expensive cleanups of perchlorate contamination - vigorously objected when the EPA proposed a 1-ppb limit for drinking water. The US military suggested a limit of 200 ppb.
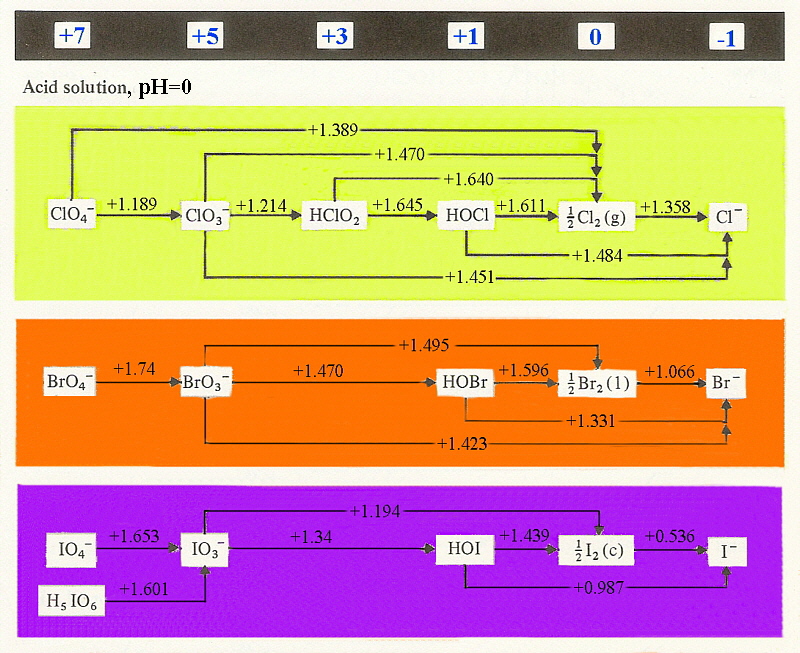
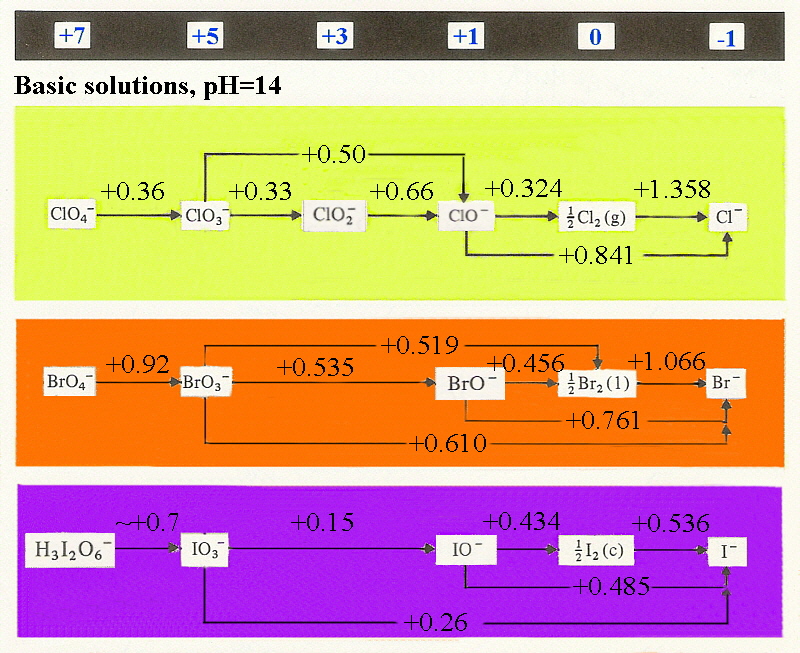
The diagrams below show the variation of redox potentials with
acid and base, It should be noted that the couples ½X2/X-
are independent of pH and together with the estimated value for F2
indicate a steady decrease in oxidising strength moving down the group.
F2 (+2.866 V) > Cl2 (+1.358 V) > Br2 (+1.066 V) > I2 (+0.536 V)
The reason the potential for difluorine must be estimated is that it is too
strong an oxidant and will oxidise water to O2 so the potential can
not be easily measured!
Given that the value listed for dichlorine is also greater than what is needed
to oxidise water (+1.229 V) this should happen as well however this is not the
case since there is a slow kinetic step in the oxidation of water.
Note as well that this is the same reason why aqueous solutions of potassium
permanganate can be prepared, although they are not stable over time.
The majority of the couples are pH dependent so that an increase in pH
causes a dramatic lowering of the E° value. This is expected since if we
consider an example where BrO3- is converted to
½Br2 by the reaction:
BrO3- + 6H+ + 5e- → ½Br2 + 3 H2O (E° = +1.495 V)
the equilibrium constant includes a term with [H+]6 so
that if the pH is changed from pH=1 to pH=14 then H+ effectively
changes by 10-14 and the estimated difference can be seen in E°
as:
ΔG° = - RTln(K)
ΔG° = - nE°F
from which just looking at the effect of pH is
~(RT/nF)ln([H+]6)
or roughly (0.0592/5) * 14 * 6
that is an effect of ~+0.99 V
Experimentally the measured E° for
BrO3- + 3H2O + 5e- → ½Br2 + 6 OH- (E° = +0.519 V)
so estimated value is +1.495 - 0.99 = +0.51 V and found is +0.519 V showing
excellent agreement.
 Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,