| 9 F 18.9984032 |
The Group 17 elements include: fluorine, chlorine, bromine, iodine and astatine and according to Housecroft and Sharpe [1] their chemistry is "probably better understood than that of any other group of elements, except the alkali metals". They share common properties such as forming singly bonded atoms as in the diatomic molecules (F2, Cl2, Br2, I2, and At2), and forming singly charged anions (F-, Cl-, Br-, I-, and At-). For this four lecture course, we will omit the chemistry of astatine since it is radioactive and the most stable isotopes of astatine have half-lives of less than a minute. As a consequence of this, only trace quantities (less than 50 ng) of astatine compounds have been investigated, severely limiting characterisation of properties. Discussions of the chemistry of the elements in Group 17 therefore focus on four elements: F, Cl, Br, and I. In 1825, the Swedish chemist Jöns Jakob Berzelius applied the term halogens (from the Greek hals, "salt," and gennan, "to form or generate") for an element that produces a salt when it forms a compound with a metal. None of the halogens are found naturally in their elemental form. They are invariably found as salts of the halide ions (F-, Cl-, Br-, and I-). [Note that Br was not discovered until 1826, after the term halogen was proposed, so it was added to the list of halogens later]. The mineral fluorspar (also called fluorite) consisting mainly of calcium fluoride, was first described as far back as 1530 by Georgius Agricola. This is a major source of fluorine in the form of fluoride ions as was cryolite (Na3AlF6) until the major deposits in Greenland ran out in 1987. In nature, chlorine is found primarily as the chloride ion, a component of the salt that is deposited in the earth or dissolved in the oceans - about 1.9% of the mass of seawater is chloride ions. Even higher concentrations of chloride are found in the Great Salt Lake in Utah, which is 9% Cl- ion by weight, the Dead Sea and in underground brine deposits. Most chloride salts are soluble in water, thus, chloride-containing minerals are usually only found in abundance in dry climates or deep underground. Common chloride minerals include halite (sodium chloride), sylvite (potassium chloride), and carnallite (potassium magnesium chloride hexahydrate). Over 2000 naturally-occurring organochlorine compounds are known. The largest bromine reserve in the United States is located in Columbia and Union County, Arkansas, U.S. China's bromine reserves are located in the Shandong Province and Israel's bromine reserves are contained in the waters of the Dead Sea. Iodine naturally occurs in the environment chiefly as a dissolved iodide in seawater, although it is also found in some minerals and soils. This element also exists in small amounts in the mineral caliche, found in Chile, between the Andes and the sea. A type of seaweed, kelp, tends to be high in iodine as well. |
| 17 Cl 35.453 |
|
| 35 Br 79.904 |
|
| 53 I 126.90447 |
|
| 85 At (210) |
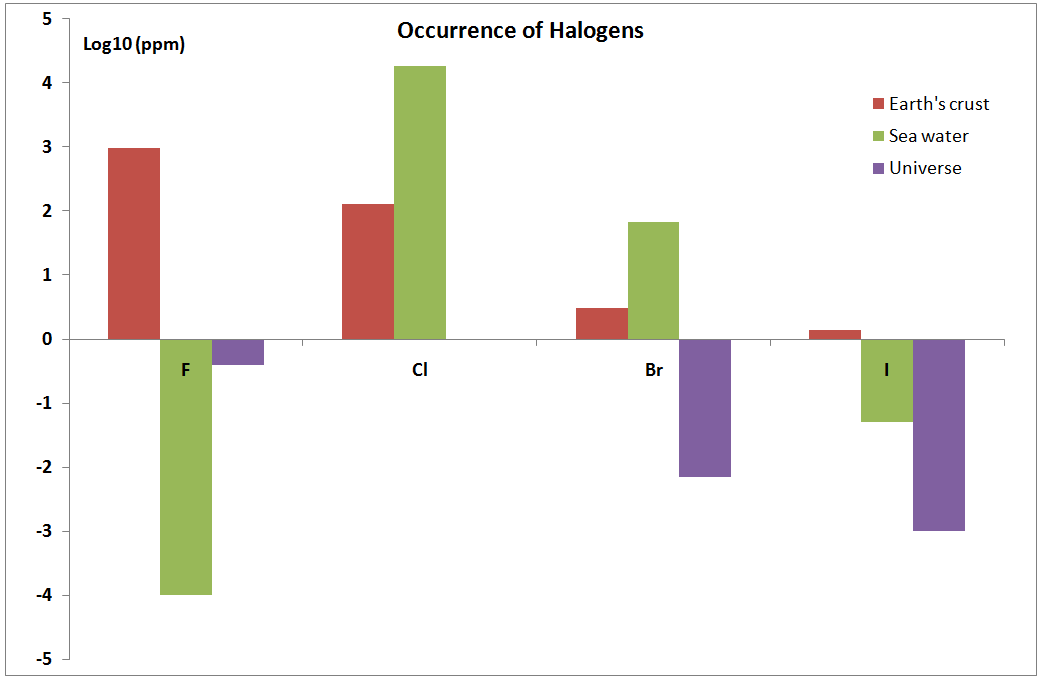
| X | Isotopes | Earth's crust (ppm) | Sea water (ppm) | Universe (ppm) |
|---|---|---|---|---|
| F | 100% - 19 | 950 | 0.0001 | 0.4 |
| Cl | (75.77%, 35) (24.23%, 37) | 130 | 18000 | 1 |
| Br | (50.69%, 79) (49.31%, 81) | 3 | 67.3 | 0.007 |
| I | 100%, 127 | 1.4 | 0.05 | 0.001 |

Difluorine (F2), is a highly toxic, colorless gas, and is highly reactive. It is so reactive that it even forms compounds with Kr, Xe, and Rn, elements once considered inert. Due to its high reactivity it is difficult to find a container in which it can be stored. F2 attacks both glass and quartz, for example, and causes most metals to burst into flame. It has to be handled in equipment built from alloys of copper and nickel where even though it still reacts with these alloys, it forms a layer of fluoride salt on the surface that protects the metal from further reaction.
Fluorine is such a powerful oxidizing agent that it can convert other elements into unusually high oxidation numbers, as in AgF2, PtF6, and IF7.
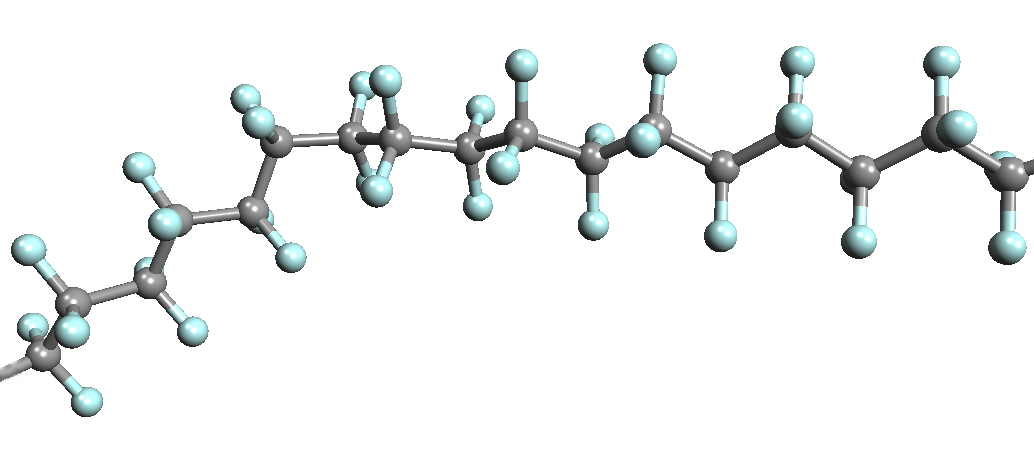
Fluorine is used in the manufacture of Teflon or PTFE poly(tetrafluoroethylene), (C2F4)n. Teflon was first prepared in 1938 by accident, since the intended product was a new refrigerant. The first non-stick frying pan was developed in France in 1954 and in the USA in 1961. Another important use is for lining of valves and gaskets that are inert to chemical reactions. This is needed in Uranium enrichment involving UF6.
Natural uranium is 99.284% 238U isotope, with 235U only constituting about 0.711% of its weight. Isotope enrichment of 235U is difficult because the two isotopes have nearly identical chemical properties, and can only be separated gradually using small mass differences. (235U is only 1.26% lighter than 238U). Gaseous diffusion was the first successful technology used to produce enrich uranium by forcing gaseous uranium hexafluoride through semi-permeable membranes. This produced a slight separation between the molecules containing 235U and 238U.
Large amounts of fluorine were consumed each year to make the "Freons" (such as Freon-12, CCl2F2) used in refrigerators. CFCs (chlorofluorocarbons) and HCFCs hydrochlorofluorocarbons) were produced by halogen exchange starting from chlorinated methanes and ethanes. For example, the synthesis of chlorodifluoromethane from chloroform:
Chlorine (Cl2) is a highly toxic gas with a pale yellow-green color and is a very strong oxidizing agent.
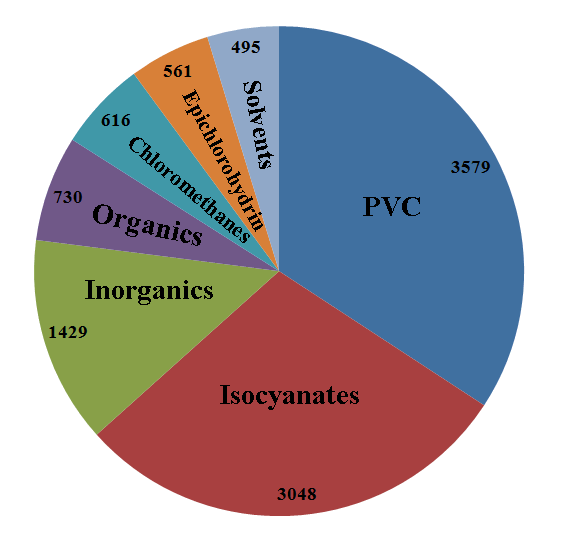 Chlorine useage in Europe, 2008 (/kt) [6] |
PVC - door and window frames, piping etc, (34.2%) Isocyanates and Oxygenates - upholstery, insulation, pesticides, (29.1%) Inorganics - disinfectants, water treatment, (13.7%) Organics - detergents, herbicides, insecticides, (7.0%) Chloromethanes - silicon rubbers, decaffeination, paint strippers, cosmetics, (5.9%) Epichlorohydrin - epoxy resins, printed circuits, sports boats, (5.4%) Solvents - metal degreasing, adhesives, (4.7%) |
PVC is the third most widely used plastic material in the world, after polyethylene and polypropylene. At the global level, demand for PVC exceeds 35 million tonnes per annum and it is in constant growth (+5% on global average), with higher growth rates in the developing countries. PVC is durable, easy to clean, stain resistant, lightweight, corrosion resistant and needs no maintenance. [7]
Chlorine has been used commercially as a disinfectant and as a bleaching agent. Chlorine was first used as a disinfectant for drinking water in the late 19th century as a means of controlling the spread of water-borne diseases such as typhoid, cholera, dysentery and gastro-enteritis. In the USA in 1900, annual deaths from cholera totalled 25,000 but following the introduction of chlorination, this figure had fallen to fewer than 20 by 1960! In 1991, a misinterpretation of US law resulted in the Peruvian government voluntarily suspending chlorination of water supplies. The resulting cholera epidemic spread to neighbouring countries causing an estimated 1 million cases of cholera and more than 10,000 deaths.[6]
Chemical pulp bleaching aims to remove coloured residual lignin from the pulp and increase its brightness, brightness stability and cleanliness while preserving the strength (cellulose integrity) and carbohydrate yield (cellulose and hemicellulose) of the unbleached fibre, with due regard for potential effects on the environment. [8]
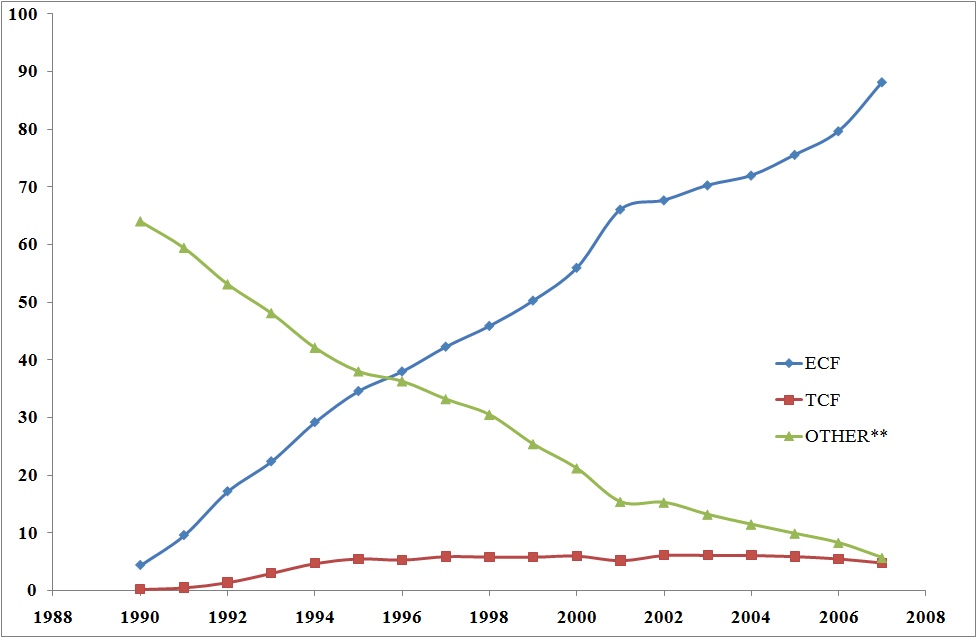 World use of bleached pulp, 1990-2007 (/Mt) [9] |
ECF - Elemental Chlorine-Free (ECF) pulp, bleached with
chlorine dioxide. TCF - Totally Chlorine-Free pulp, a small 5% niche market. other - this includes dichlorine |
ECF, using ClO2, accounts for 99% of the bleached chemical pulp production in the USA, while dichlorine was essentially phased out in 2001 in compliance with rules from the Environmental Protection Agency. Cl2 was found to contribute to the formation of perchlorodibenzodioxins PCDD and perchlorodibenzofurans PCDF. A number of these are highly toxic, persistent (lasting for years or even decades before degrading into less dangerous forms), highly volatile and liable to accumulate in fatty tissue, hence they are called persistent organic pollutants (POP).
For example, tetrachlorodibenzodioxin and hexachlorodibenzofuranSome 85% of pharmaceuticals contain or are manufactured using chlorine, including products to treat Aids, allergies, arthritis, cancer, depression, diabetes, heart disease, hypertension, infections, pneumonia and ulcers. An example is the natural antibiotic vancomycin, an effective medicine in fighting hospital Staphylococcus infections.[6]
Bromine (Br2) is a reddish-orange liquid with an unpleasant, choking odor. The name of the element is derived from the Greek stem bromos, "stench." Bromine is used to prepare flame retardants, fire-extinguishing agents, sedatives and insecticides.
There are approximately 75 different brominated flame
retardants (BFRs) of which Deca-BDE, TBBPA and HBCD are the three
main commercial products.
Deca-BDE
1,2,3,4,5-pentabromo-6-(2,3,4,5,6-pentabromophenoxy)benzene
is a highly effective brominated flame retardant which is used to
prevent fires in plastics for electrical and electronic equipment
as well as in contract textiles.
TBBPA 2,2',6,6'-Tetrabromo-4,4'-isopropylidenediphenol is the
largest BFR in terms of production. 70% is used as a reacted
flame retardant in polymers like epoxy resins in electrical and
electronic equipment and 20% is used as an additive to
plastics.
HBCD 1,2,5,6,9,10-hexabromocyclododecane has been used for
many years mainly in thermal polystyrene insulation foams and
applied in the back-coating of textiles for upholstered
furniture. [10]
Marine organisms are the main source of naturally occuring organobromine compounds. Perhaps the most famous example and certainly oldest of these is a dyestuff that has been used by humans since the 18th century BC, Tyrian purple. The dye, Tyrian purple - 6,6'-dibromoindigo was extracted from the the marine gastropods Murex brandaris and the purple silks produced were a status symbol of the Byzantium imperial court.
Iodine is an intensely colored solid with an almost metallic luster. The solid is relatively volatile, and it sublimes when heated to form a violet-colored gas. Iodine has been used for many years as a disinfectant in "tincture of iodine." Iodine compounds are used as catalysts, drugs, and dyes. Silver iodide (AgI) played an important role in the photographic process and in attempts to seed clouds to make rain. Iodide is also added to salt to protect against goiter, an iodine deficiency disease characterized by a swelling of the thyroid gland.
Among dyes that have a high iodine content is erythrosine B (food red-colour additive E127 (Federal Food, Drug and Cosmetic Act, Red #3)
 Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,