Unit - Chemistry of Garments: Cellulose
Fibres
For some background material see the
Free Text book
The Basics of General, Organic, and Biological Chemistry by David
W. Ball, John W. Hill, Rhonda J. Scott.
Chapter 16, section 7
Polysaccharides
The polysaccharides are the most abundant carbohydrates in nature
and serve a variety of functions, such as energy storage or as
components of plant cell walls. Polysaccharides are very large
polymers composed of tens to thousands of monosaccharides joined
together by glycosidic linkages. The three most abundant
polysaccharides are starch, glycogen, and cellulose. These three
are referred to as homopolymers because each yields only one type
of monosaccharide (glucose) upon complete hydrolysis.
Starch
Starch is the most important source of carbohydrates in the human
diet and accounts for more than 50% of our carbohydrate intake.
It occurs in plants in the form of granules, and these are
particularly abundant in seeds (especially the cereal grains) and
tubers, where they serve as a storage form of carbohydrates. The
breakdown of starch to glucose nourishes the plant during periods
of reduced photosynthetic activity. (Irish) Potatoes are often
considered as a "starchy" food, yet other plants contain a much
higher percentage of starch (potatoes 15%, wheat 55%, corn 65%,
and rice 75%). Commercial starch is a white powder.
Glycogen
Glycogen is the energy reserve carbohydrate of animals.
Practically all mammalian cells contain some stored carbohydrates
in the form of glycogen, but it is especially abundant in the
liver (4%-8% by weight of tissue) and in skeletal muscle cells
(0.5%-1.0%). Like starch in plants, glycogen is found as granules
in liver and muscle cells. When fasting, animals draw on these
glycogen reserves during the first day without food to obtain the
glucose needed to maintain metabolic balance.
Note.
About 70% of the total glycogen in the body is stored in muscle
cells. Although the percentage of glycogen (by weight) is higher
in the liver, the much greater mass of skeletal muscle stores a
greater total amount of glycogen.
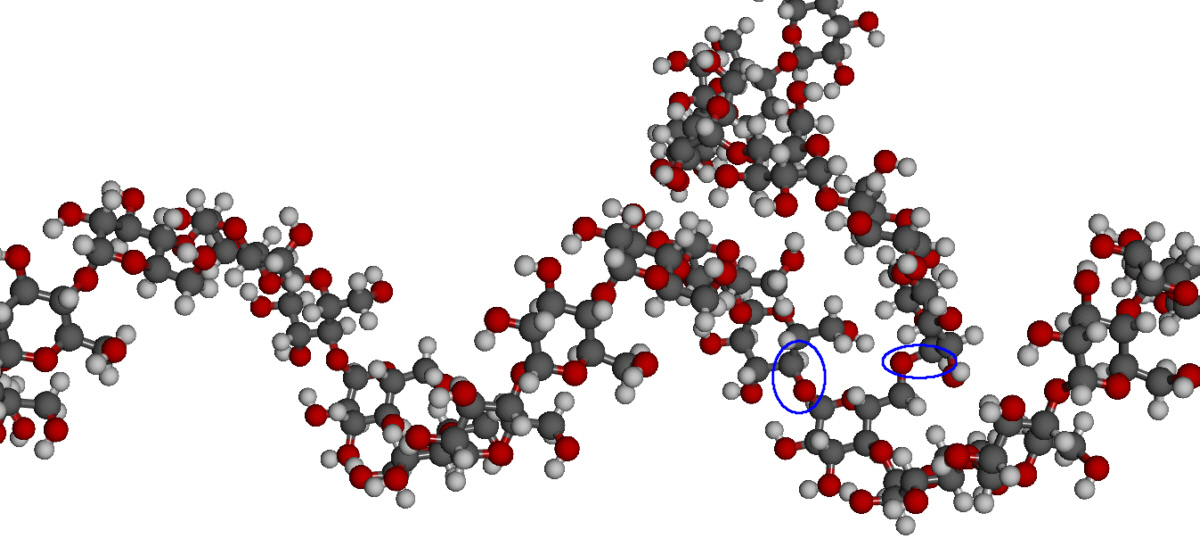
Cellulose
Cellulose, a fibrous carbohydrate found in all plants, is the
structural component of plant cell walls. Because the earth is
covered with vegetation, cellulose is the most abundant of all
carbohydrates, accounting for over 50% of all the carbon found in
the vegetable kingdom. Cotton fibrils and filter paper are almost
entirely cellulose (about 95%), wood is about 50% cellulose, and
the dry weight of leaves is about 10%-20% cellulose. The largest
use of cellulose is in the manufacture of paper and paper
products. Although the use of noncellulose synthetic fibers is
increasing, rayon (made from cellulose) and cotton still account
for over 70% of textile production.
Cellulose and starch are based on the same repeating monomeric unit
(D-glucose). The properties of these polysaccharides however are
very different.
- The orientation of the 1,4-glycosidic linkages is beta and
alpha respectively between the repeating glucose units. For
cellulose, this results in the neighbouring units being rotated
by 180° with respect to each other and produces a long,
straight, rigid molecule.
- There are no side chains in cellulose as there are in starch.
The absence of side chains allows these linear molecules to lie
close together.
- The presence of the -OH groups, as well as the oxygen atom in
the ring, provides many opportunities for hydrogen bonds to form
between adjacent chains. These hold the chains firmly together
side-by-side to form microfibrils with high tensile strength.
This strength is important in plant cell walls, where the
microfibrils are meshed into a carbohydrate matrix, conferring
rigidity to plant cells.
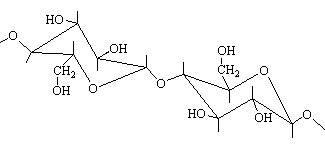
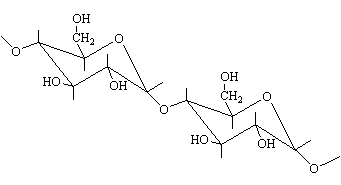
beta and alpha 1,4- links between glucose units in cellulose and
starch
The resulting polymeric structures are such that cellulose gives
rise to linear arrangements while starch is non-linear and
branched.
Natural starches are a mixture of two polymers: amylose (10%-30%)
and amylopectin (70%-90%). Amylose is not branched and is coiled
like a spring, with six glucose monomers per turn. When coiled in
this fashion, amylose has sufficient room in its core to
accommodate the tri-iodide ion. The characteristic blue-violet
colour that appears when starch is treated with iodine is due to
the formation of the amylose-tri-iodide complex. This colour test
is sensitive enough to detect even minute amounts of starch in
solution.
tri-iodide ions inside coils of glucose in amylose, see
BioTopics
Amylopectin on the
other hand is a branched-chain polysaccharide where in addition
to the α-1,4-glycosidic bonds there exist the occasional
α-1,6-glycosidic bonds, which are responsible for the
branching. Branching occurs about every 24-30 glucose units.
Since the helical structure of amylopectin is disrupted by
branching, the reaction of iodine with amylopectin does not
produce the deep blue colour but instead a less intense reddish
brown colour.
Cellulose fibres.
The presence of linear chains of thousands of glucose units
linked together allows a great deal of hydrogen bonding between
OH groups on adjacent chains, causing them to pack closely into
cellulose fibers. As a result, cellulose exhibits little
interaction with water or any other solvent. Cotton and wood, for
example, are completely insoluble in water and have considerable
mechanical strength. Since cellulose does not have a helical
structure like amylose, it does not bind to iodine to form a
coloured product.
Strands of cellulose
Note.
Humans are unable to metabolize cellulose as a source of glucose
since our digestive juices lack the enzymes that can hydrolyze
the glycosidic linkages. So although we can digest potatoes, we
cannot process grass. However, certain microorganisms can digest
cellulose because they contain the enzyme cellulase, capable of
catalysing the hydrolysis of cellulose. The presence of these
microorganisms in the digestive tracts of herbivorous animals
(such as cows, horses, and sheep) allows these animals to degrade
the cellulose from plant material into glucose for energy.
Termites also contain cellulase-secreting microorganisms and thus
can subsist on a wood diet.
While on the
subject of the digestion of saccharides, there has been
much debate about the use of
high-fructose corn syrup (HFCS) and the increase
in the number of people with obesity.
Vegetable Cellulose
Fibres
- fibre occurring on the seed (raw cotton, java cotton)
- phloem fiber (flax, ramie, hemp, jute)
- tendon fibre from stem or leaves (manila hemp, sisal hemp
etc)
- fibre occurring around the trunk (hemp palm)
- fibre of fruit/nut shells (coconut fibre - Coir)
cotton and linen are the most important among them and are forms
of cellulose.
Cotton
Cotton has been used as a textile fibre for thousands of years
with India being generally considered as the birthplace of cotton
cloth. Cotton is a hair attached to the seed of several species
of the genus Cossypium, a shrub up to 2 metres in height,
indigenous to nearly all tropical regions but growing best near
the sea, lakes or large rivers where there is a warm humid
climate and sandy soil. Cotton or cotton mixed with synthetic
polymers provides most of the clothing in the world. It is used in
making the finest garments suited to hot or cold weather,
bed-sheets, and for worldwide popular jeans. Each cotton fibre
has 20-30 layers of cellulose built up in an orderly series of
spring-like spirals. These fibres bring out certain
characteristics like absorbency wet-strength, softness and
durability in cotton clothing.
See textile
manufacturing for the steps involved from growing cotton to
the production of cloth. The finishing-processing of textiles
involves:
Sizing/desizing
Depending on the size that has been used, the cloth may be
steeped in a dilute acid and then rinsed, or enzymes may be used
to break down the size.
Scouring
Scouring, is a chemical washing process carried out on cotton
fabric to remove natural wax and non-fibrous impurities (eg the
remains of seed fragments) from the fibres and any added soiling
or dirt. Scouring is usually carried in iron vessels called
kiers. The fabric is boiled in an alkali, which forms a soap with
free fatty acids (saponification). A kier is usually enclosed, so
the solution of sodium hydroxide can be boiled under pressure,
excluding oxygen which would degrade the cellulose in the fibre.
If the appropriate reagents are used, scouring will also remove
size from the fabric although desizing often precedes scouring
and is considered to be a separate process known as fabric
preparation. Preparation and scouring are prerequisites to most
of the other finishing processes. At this stage even the most
naturally white cotton fibres are yellowish, and bleaching, the
next process, is required.
Bleaching
Bleaching improves whiteness by removing natural coloration and
remaining trace impurities from the cotton; the degree of
bleaching necessary is determined by the required whiteness and
absorbency. Cotton being a vegetable fibre will be bleached using
an oxidizing agent, such as dilute sodium hypochlorite or dilute
hydrogen peroxide. If the fabric is to be dyed a deep shade, then
lower levels of bleaching are acceptable, for example. However,
for white bed sheetings and medical applications, the highest
levels of whiteness and absorbency are essential.
Mercerising
A further possibility is mercerizing during which the fabric is
treated with caustic soda solution to cause swelling of the
fibres. This results in improved lustre, strength and dye
affinity. Cotton is mercerized under tension, and all alkali must
be washed out before the tension is released or shrinkage will
take place. Mercerizing can take place directly on grey cloth, or
after bleaching.
Many other chemical treatments may be applied to cotton fabrics
to produce low flammability, crease resist and other special
effects. Some other important treatments include
dyeing and
printing.
Linen
Linen is woven from yarn made from the fibres of the flax plant, a member of
the genus Linum in the family Linaceae. The flax plant has blue
or white flowers and grey-green stems, and it grows to a height
of about 1 metre. It grows well in temperate and fairly equable
climates, free from very heavy rains, although moist winds during
the growing season are good.
The following comment is taken from BelovedLinens.net
The various processes employed in the preparation of the plant in
Egypt are admirably depicted on the enduring walls of their
ancient palaces, temples, and tombs, by the skilful hand of the
artist. Drawings of the various implements employed; of the
people in the act of sowing the seed; pulling the plant; carrying
water to fill wooden vats, evidently for the purpose of steeping
the Flax; putting it through the several processes requisite to
produce the fiber; spinning it into yarn; and weaving the yarn
into cloth, are all distinctly portrayed.
This depiction is
from the Theban Tomb located in Deir el-Medina, part of the
Theban Necropolis, on the west bank of the Nile, opposite to
Luxor. It is the burial place of the Ancient Egyptian official,
Sennedjem and his family. He is seen pulling the flax out by hand
(rather than cutting), after which it is tied in bundles and
carried off the field on the back of oxen.
The steeping and the subsequent process of beating the stalks
with mallets shown on the walls of the tombs, illustrates the
following passage of Pliny upon the same subject:-" The
stalks themselves are immersed in water, warmed by the heat of
the sun, and are kept down by weights placed upon them; for
nothing is lighter than Flax. The membrane or rind, becoming
loose, is a sign of their being sufficiently macerated. They are
then taken out and repeatedly turned over in the sun until
perfectly dried, and afterwards beaten by mallets on stone slabs.
That which is nearest the rind is called tow, inferior to the
inner fibers, and fit only for the wicks of lamps. It is combed
out with iron hooks, until all the rind is removed. The inner
part is of a whiter and finer quality. Men are not ashamed to
pre-pare it. . . . After it is made up into yarn it is polished
by striking it frequently on a hard stone, moistened with water.
When woven into cloth it is again beaten with clubs, being always
improved in proportion as it is beaten."
Canadian video showing the processing of flax to linen
Irish
Linen production
Apart from clothes, linen has been used in the making of tea
towels, bath-towels and linen because of its strong capacity to
absorb moisture.
Over 42 km (26 miles) of flax and hemp rope was required to rig the HMS Victory,
with the largest rope being 47 cm (19 inches) in circumference. A set of canvas
sails for the HMS Victory comprised of 37 sails providing a total sail area
of 5,428 m2 (6,500 square yards). An additional 23 sails were
carried on board as spares.
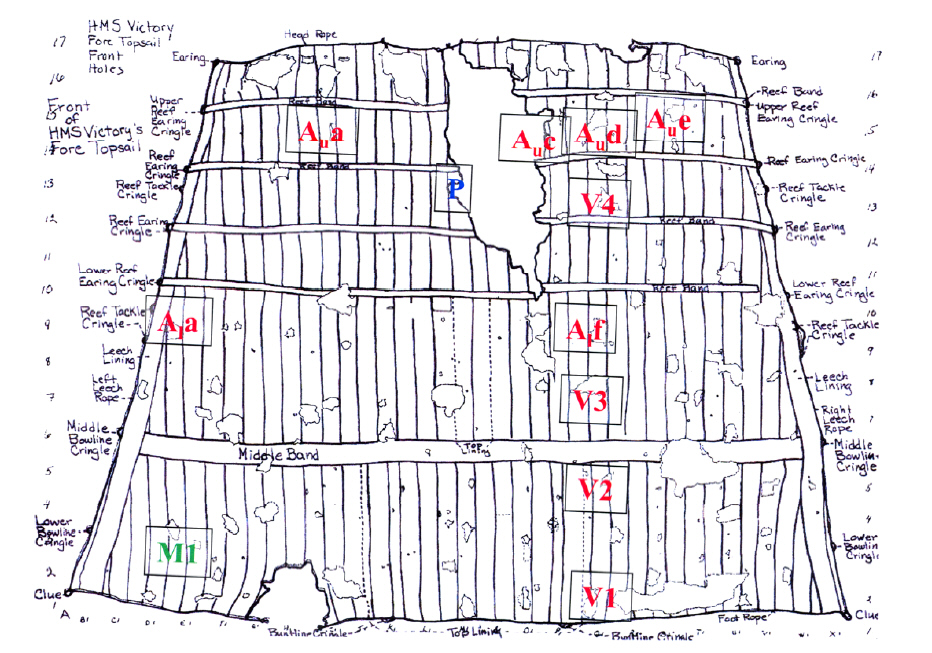
The largest single artefact left from the Battle of Trafalgar is
the fore-topsail of HMS Victory, the flagship of Admiral Lord
Nelson. Measuring 24m (80 ft) at its base, 17 m (54 ft) at the
head and 17m (54 ft) deep, this one sail covers an area of 336
m2 (402 square yards) and weighs roughly 370 kg.
If you want to build your own 16m replica you can get the plans
from
HobbyNuts
The sail was initially made in the Sail Loft at Chatham when HMS Victory was
completing her repair in 1803. The Dundee weavers who used to manufactured the
bolts of cloth for the Navy during this period would have spent around
1,200 hours to stitch the top sail together and they remained on board the ship
until it was returned to the Sail Loft for repairs, after the battle in 1806.
This surviving topsail is pot marked by over 80 holes and tears in the canvas
sustained both in the battle and afterwards by 19th century souvenir hunters.
It was exhibited in No 4 Boathouse in the Royal Naval Dockyard during the
International Festival of the Sea in 1998, before being removed for further
restoration. This was the first time the sail had been hoisted since the
Battle of Trafalgar. To preserve the sail for the
future it is now being stored in environmentally controlled conditions in
Store 10, in the Portsmouth Historic Dockyard. it is arguably
Britain's foremost maritime textile treasure.
Prior to its display at the
bicentennial exhibition in 2005, the sail was analysed by
Infra-red and
Raman spectroscopy and
conservationists did some repair and preservation work. Complementary
tensile tests were completed on loose yarn from around the damaged areas.
The mechanical data and Raman spectral comparisons suggested a good
correspondence between the historic sailcloth and surrogate
specimens.
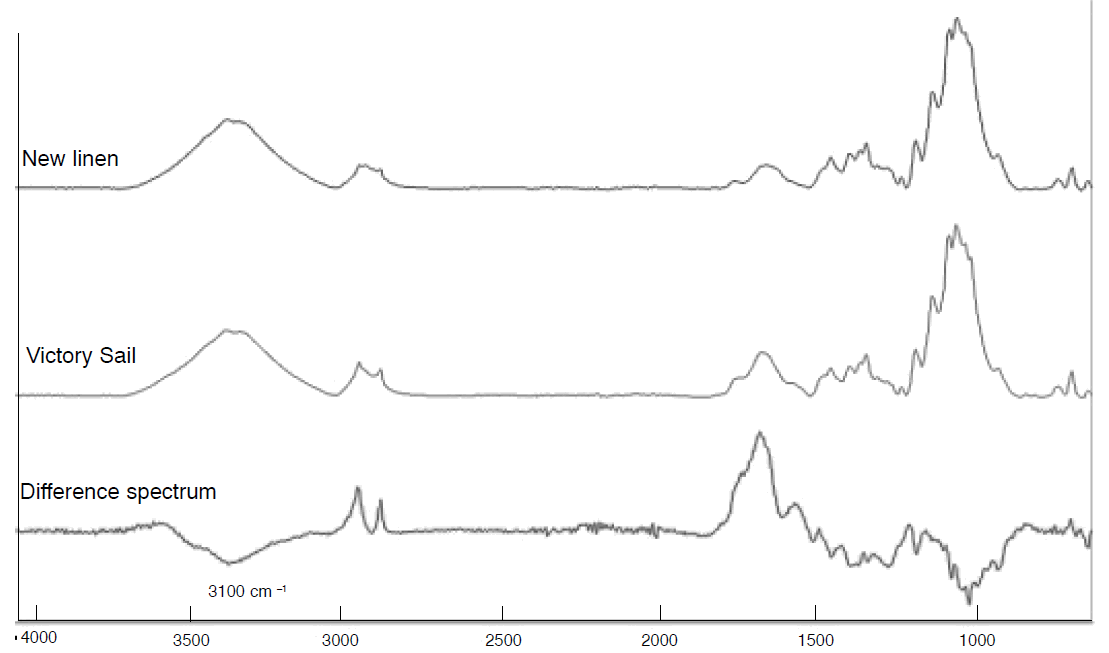
FTIR of sample from the HMS Victory fore-sail compared to new linen.
The deterioration over the years has come in part from the aerial oxidation
of the alcohol groups to carboxylic acids. This can be seen in the difference
spectrum calculated from the IR spectrum of fresh linen compared to that of the sail.
The band at 1720 cm-1 being due to acid formation (C=O) and the
negative band at ~3100 cm-1 showing the loss of the OH groups.
The two bands at 2930 cm-1 and 2850 cm-1 show that the
sail has gained some oils or waxes that were not originally present.
These notes adapted from:
HMS Victory conservation
and HMS Victory information
Semi-synthetic cellulose based fibres
Artificial
silk
Artifical silk is frequently used as a synonym for rayon.
The first successful artificial silks were developed in the 1890s
from cellulose fiber and marketed as "artificial silk" or
"viscose", a trade name for a specific manufacture. In the U.S.A.
in 1924, the name of the fiber was officially changed to rayon,
although the term viscose continued to be used in Europe. The
term "viscose rayon" refers to the same material.
Prior to that "nitro-cellulose"
had been prepared but was found to be impractical due to its
instability and flammability. It has alternatively been called
"guncotton" and is not a nitro compound but has nitrate groups
replacing three of the OH groups.
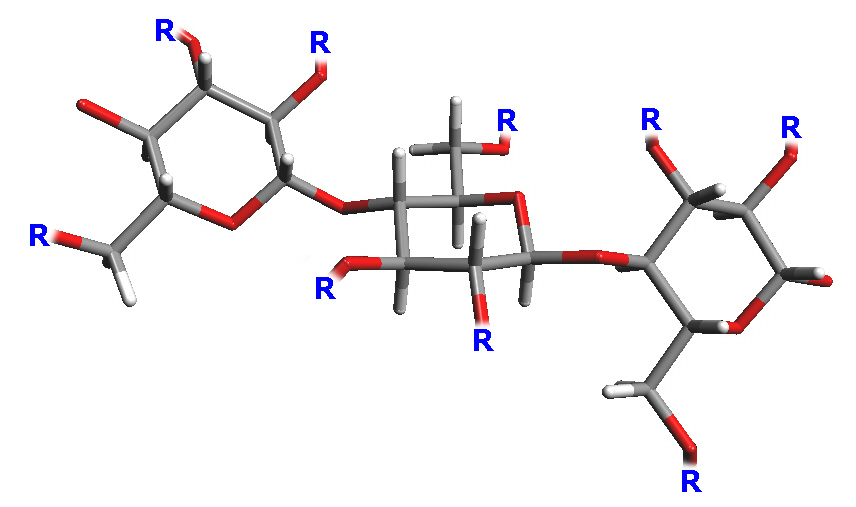
The R groups replacing the OH's, might be nitrate,
CS
2Na, acetates or other substituents
Rayon
For some background reading see the J Chem Educ article by Prof
G.B. Kauffman, Rayon - the first
semi-synthetic fibre product
Rayon is a manufactured regenerated cellulose fiber. Because it
is produced from naturally occurring polymers, it is neither a
truly synthetic fiber nor a natural fiber; it is a semi-synthetic
or artificial fiber. Rayon is known by the names viscose rayon
and artifical silk in the textile industry.
Most linear celluloses can be dissolved in solvents capable of breaking
the strong hydrogen bonds, such as aqueous inorganic acids, calcium
thiocyanate, zinc or lithium chlorides, dimethyldibenzylammonium hydroxide,
ammoniacal cadmium hydroxide, and ammoniacal copper hydroxide
(Schweizer's reagent). Only high-molecular weight native cellulose, which is
insoluble in 17.5% aqueous sodium hydroxide and which is called
α-cellulose, can be used in producing rayon. The only materials
containing a high enough percentage of α-cellulose for making rayon
are cotton (plants of the genus Gossypium) and some types
of wood pulp.
In 1857, the Swiss chemist, Matthias Eduard
Schweizer, discovered that copper(II) ammonia
solutions could dissolve cellulose. He reported that...
This possesses to an extraordinary degree the power to
dissolve plant fiber at ordinary temperature.
If purified cotton is covered with the blue solution, it soon
assumes a gelatinous, slippery state, the fibers separate and
disappear. and after some working up with a glass rod everything
is converted into a slimy liquid..... If an insufficient amount
of solution is used, a portion of the fibres still remains
visible; however, if an excess of the solution is added and
shaken, an almost clear blue solution is obtained, which, after
it has been diluted with water, can be filtered.
If the filtered solution is supersaturated with hydrochloric
acid, a voluminous white precipitate is formed, which, collected
on a filter, has the exact appearance of moist aluminum
hydroxide. This substance, unorganized, to be sure, but not
changed essentially in its chemical nature, appears to be
cellulose. If the gelatinous precipitate, freed completely from
salts by washing, is dispersed in water, and potassium iodide and
afterward some chlorine water is added, the substance is colored
brown [not blue], proof that it is neither starch nor a
starch-containing substance.
On being dried on a water bath, the precipitate shrinks together
strongly and leaves behind a horny, translucent, brittle mass,
which is similar to dried paste but has no taste at all and does
not stick to the teeth. Heated in the air, the substance burns
without leaving a residue.. . .
If the fiber solution is painted on a glass plate and allowed to
dry on it, a thin, bluish white coat remains, which adheres
solidly to the glass....
Production method for Viscose Rayon
Regular rayon (or viscose) is the most
widely produced form of rayon. This method of rayon production
has been utilized since the early 1900s and it has the ability to
produce either filament or staple fibers.
The process is as follows:
- Cellulose: Production begins with
processed cellulose
- Immersion: The cellulose is
dissolved in caustic soda:
(C6H10O5)n + nNaOH
→ (C6H9O4ONa)n +
nH2O
- Pressing: The solution is then
pressed between rollers to remove excess liquid
- White Crumb: The pressed sheets are
crumbled or shredded to produce what is known as "white
crumb"
- Aging: The "white crumb" aged
through exposure to oxygen
- Xanthation: The aged "white crumb"
is mixed with carbon disulfide in a process known as Xanthation,
the aged alkali cellulose crumbs are placed in vats and are
allowed to react with carbon disulfide under controlled
temperature (20 to 30°C) to form cellulose xanthate:
(C6H9O4ONa)n +
nCS2 →
(C6H9O4O-SC-SNa)n
- Yellow Crumb: Xanthation changes
the chemical makeup of the cellulose mixture and the resulting
product is now called "yellow crumb"
- Viscose: The "yellow crumb" is
dissolved in a caustic solution to form viscose
- Ripening: The viscose is set to
stand for a period of time, allowing it to ripen:
(C6H9O4O-SC-SNa)n +
nH2O →
(C6H10O5)n +
nCS2 + nNaOH
- Filtering: After ripening, the
viscose is filtered to remove any undissolved particles
- Degassing: Any bubbles of air are
pressed from the viscose in a degassing process
- Extruding: The viscose solution is
extruded through a spinneret, which resembles a shower head with
many small holes
- Acid Bath: As the viscose exits the
spinneret, it lands in a bath of sulfuric acid, resulting in the
formation of rayon filaments:
(C6H9O4O-SC-SNa)n +
½nH2SO4 →
(C6H10O5)n +
nCS2 + ½nNa2SO4
- Drawing: The rayon filaments are
stretched, known as drawing, to straighten out the fibers
- Washing: The fibers are then washed
to remove any residual chemicals
- Cutting: If filament fibers are
desired the process ends here. The filaments are cut down when
producing staple fibers
More information is available from the CIRFS: European Man-Made
Fibres Association on
Viscose
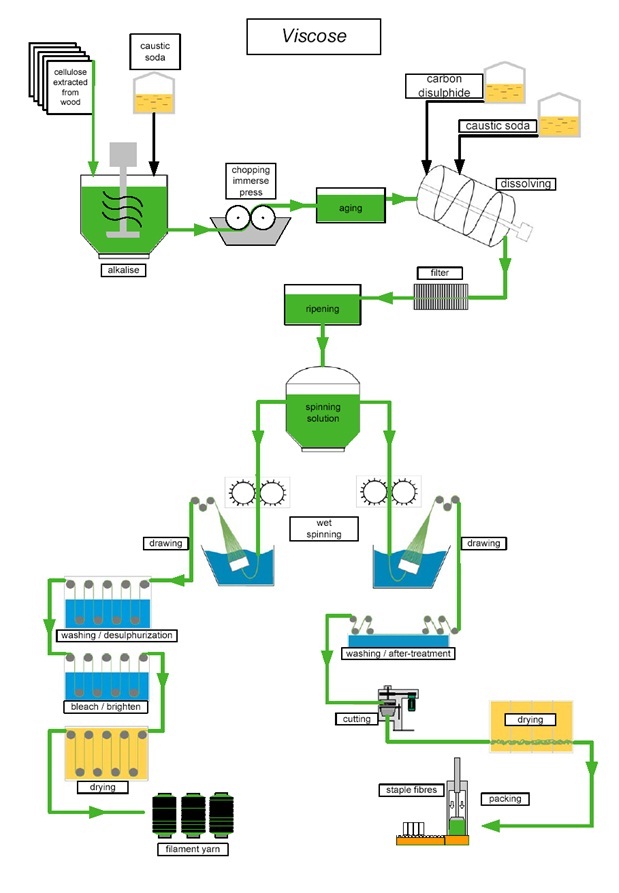
Steps involved on the production of Viscose Rayon
In 2010 the Federal Trade Commission (FTC) issued a statement
concerning the advertising of fabric as being derived from bamboo
with the implication that they were environmentally friendly.
see Bamboo-zled by bamboo
Rayon is made from plants and trees, bamboo included, but it
must go through a chemically-intensive process that results in a
considerable amount of pollution -- a fact that runs contrary to
the environmentally-friendly claims of bamboo clothing.
Clothing made using bamboo fibers is not of the same silky smooth
consistency as those made with rayon, the FTC said. Once bamboo
or any other tree is turned into rayon, it is misleading to claim
it is anything other than rayon because of the chemical bath used
to convert the material from a natural product into rayon. It can
be called "rayon made from bamboo," which isn't misleading as
long as you understand there's nothing environmentally friendly
about rayon.
Cellophane
Cellophane is a thin, transparent sheet made from (viscose)
regenerated cellulose by extruding the solution through a narrow
slit into an acid bath. It has low permeability to air, oil,
grease and bacteria which makes it useful for food packaging.
Cellulose
triacetate
Cellulose triacetate (CTA) was first commercially produced in the
USA in 1954 and is derived from cellulose by acetylating
cellulose with acetic acid and/or acetic anhydride.
Characteristics of textiles containing CTA
- Shrink resistant
- Wrinkle resistant
- Easily washable
- Generally washable at high temperatures
- Maintains creases and pleats well
In late 2010 Eastman Chemical Manufacturer announced a 70%
increase in output to supply increasing demand for the chemical's
use as an intermediate in the production of polarized films for
liquid crystal displays (LCD)s.
Cellulose
acetate phthalate
Cellulose acetate phthalate (CAP) is commonly prepared by the
reaction of a partially substituted cellulose acetate (CA) with
phthalic anhydride in a solvent such as acetic acid, acetone, or
pyridine in the presence of a basic catalyst. The basic catalysts
employed are anhydrous sodium acetate when the solvent is acetic
acid, amines when using acetone, while pyridine is able to act as
both solvent and base.
The structures obtained result in about half of the hydroxyls
being esterified with acetyls, a quarter are esterified with one
or two carboxyls of a phthalic acid, and the remainder are
unchanged.
Recently (CAP), a pharmaceutical excipient used for enteric film
coating of capsules and tablets, was found to inhibit infection
by the human immunodeficiency virus type 1 (HIV-1) and several
herpesviruses. See Pub Med
for a copy of the article.
Acknowledgements.
Much of the information in these course notes has been sourced from
Wikipedia under the Creative Commons License. Students taking this course
will be expected to contribute to Wikipedia as a part of their course
assignments.
Continue to Animal Fibres or
return to the CHEM2402 course
outline.

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica
Created August 2011. Links checked and/or last
modified 27th September 2013.
URL
http://wwwchem.uwimona.edu.jm/courses/CHEM2402/Textiles/Veg_Fibres.html













 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,