

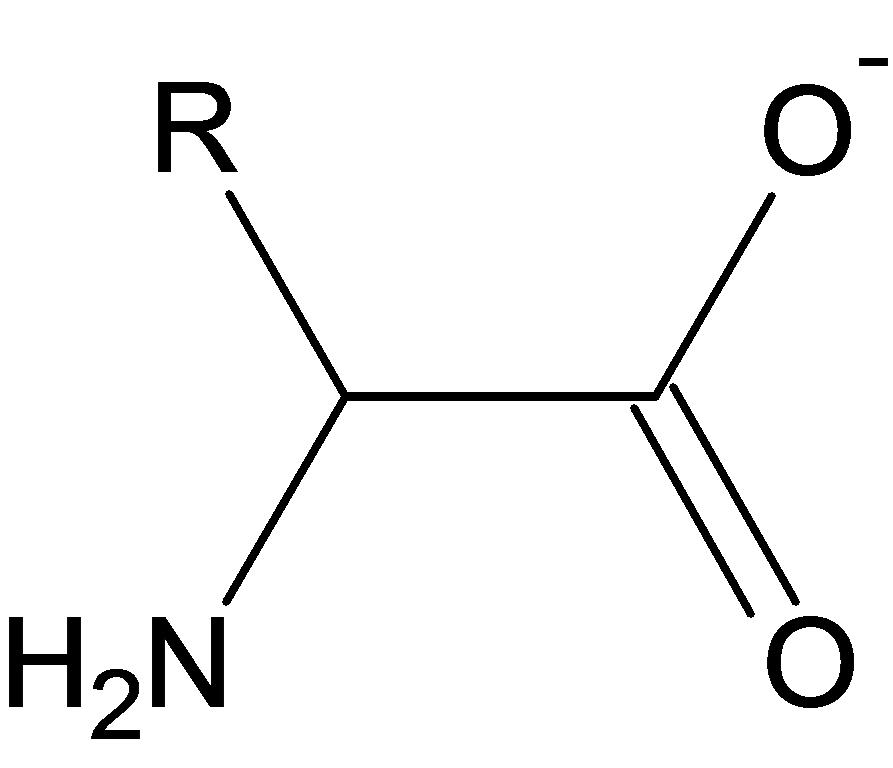
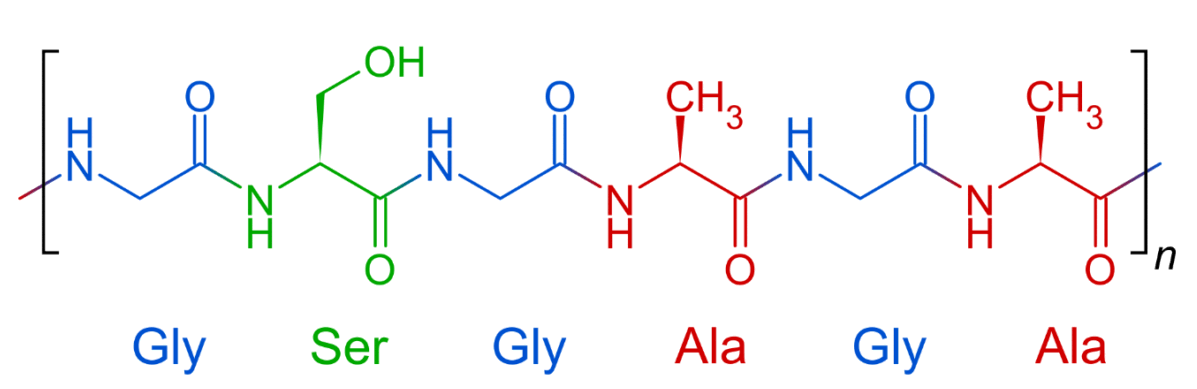
| symbol | Amino acid | Fibroin | Sericin |
|---|---|---|---|
| G | (glycine) | 45 | 14 |
| A | (alanine) | 29 | 5 |
| S | (serine) | 12 | 33 |
| Y | (tyrosine) | 5 | 3 |
| V | (valine) | 2 | 3 |
| D | (aspartic acid) | 1 | 15 |
| R | (arginine) | 1 | 3 |
| E | (glutamic acid) | 1 | 8 |
| I | (isoleucine) | 1 | 1 |
| L | (leucine) | 1 | 1 |
| F | (phenylalanine) | 1 | 1 |
| T | (threonine) | 1 | 8 |
| C | (cystine); half | 0 | 0 |
| H | (histidine) | 0 | 1 |
| K | (lysine) | 0 | 4 |
| M | (methionine) | 0 | 0 |
| P | (proline) | 0 | 1 |
| W | (tryptophan) | 0 | 0 |

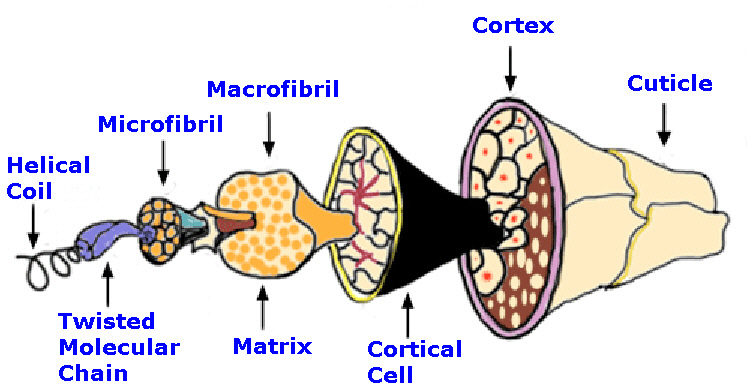
| Component | Merino Sheep Wool | Human Hair | Mohair | |||
|---|---|---|---|---|---|---|
| g 100 g-1 | mmol g-1 | g 100 g-1 | mmol g-1 | g 100 g-1 | mmol g-1 | |
| Cys | 12.02 | 1000 | 17.08 | 1422 | 9.7 | 808 |
| Glu | 14.41 | 980 | 13.02 | 885 | 15.52 | 1055 |
| Ser | 9.66 | 920 | 8.94 | 851 | 7.83 | 745 |
| Gly | 5.25 | 700 | 3.84 | 512 | 4.84 | 645 |
| Leu | 8.26 | 630 | 6.08 | 464 | 8.7 | 672 |
| Pro | 6.79 | 590 | 8.67 | 753 | 6.41 | 557 |
| Arg | 9.58 | 550 | 8.29 | 476 | 8.53 | 490 |
| Thr | 6.54 | 550 | 6.45 | 542 | 5.74 | 482 |
| Asp | 6.65 | 500 | 5.52 | 425 | 7.24 | 544 |
| Ala | 4.1 | 460 | 3.07 | 345 | 4.03 | 452 |
| Val | 5.38 | 460 | 5.73 | 490 | 7.76 | 663 |
| Tyr | 5.25 | 290 | 2.28 | 126 | 3.51 | 194 |
| Ile | 3.41 | 260 | 2.78 | 212 | 3.57 | 272 |
| Phe | 3.8 | 230 | 2.36 | 143 | 4.04 | 245 |
| Lys | 3.22 | 220 | 2.6 | 178 | 3.26 | 223 |
| Trp | 1.43 | 70 | ||||
| His | 1.02 | 66 | 0.96 | 62 | 1.09 | 70 |
| Met | 0.52 | 39 | ||||
| Hyl | 0.16 | 10 | ||||
| Ammonia | 1.2 | 750 | 1.28 | 797 | 1.27 | 793 |
| N% | 16.35 | 16.5 | 16.6 | |||
| S% | 3.65 | 5.1 | 3.0 | |||

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,